
 |
||||||||

| Tracks 1-15 | ||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-2
![]() DR LOVE: Would you discuss the Phase III randomized trial of lapatinib in combination with letrozole as first-line therapy for postmenopausal women with ER/PR-positive metastatic breast cancer?
DR LOVE: Would you discuss the Phase III randomized trial of lapatinib in combination with letrozole as first-line therapy for postmenopausal women with ER/PR-positive metastatic breast cancer?
![]() DR PEGRAM: The trial was conducted in a patient population not selected
for HER2 status, but it was statistically powered to evaluate the subset with HER2-positive disease as the primary endpoint. In fact, the statistical plan
called for the analysis of that subset first, and only if that reached statistical
significance would they analyze the intent-to-treat population, which included
all the patients with HER2-negative disease (Johnston 2008).
DR PEGRAM: The trial was conducted in a patient population not selected
for HER2 status, but it was statistically powered to evaluate the subset with HER2-positive disease as the primary endpoint. In fact, the statistical plan
called for the analysis of that subset first, and only if that reached statistical
significance would they analyze the intent-to-treat population, which included
all the patients with HER2-negative disease (Johnston 2008).
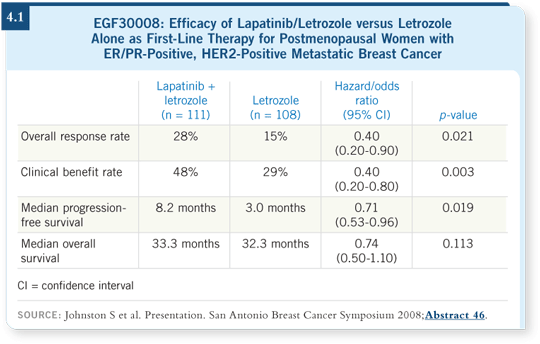
The result was a statistically significant increase in progression-free survival and response rate with lapatinib/letrozole compared to letrozole alone among patients with ER/PR-positive, HER2-positive disease. The overall survival data are immature, with an interesting trend that did not reach statistical significance — a longer data-capture period is required (Johnston 2008; [4.1]).
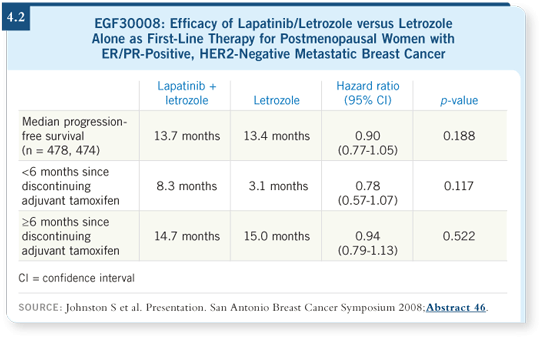
As the results were positive for patients with HER2-positive disease, they studied the entire cohort of 1,286 subjects, most of whom had ER/PR-positive, HER2-negative disease. In the group with HER2-negative disease, no efficacy signal was noted (Johnston 2008; [4.2]).
Another interesting twist in the statistical plan stipulated evaluating those patients who experienced disease progression within six months of discontinuing adjuvant tamoxifen. In that portion, which by protocol definition was an endocrine-resistant subpopulation of the patients with HER2-negative disease, a statistically nonsignificant increase in progression-free survival was observed with lapatinib/letrozole (Johnston 2008; [4.2]).
This raises the possibility that something of note might be occurring in endocrine-resistant, HER2-negative disease, which would require confirmation in prospective randomized trials. It’s not practice changing in this population at this point. But the primary endpoint of the study in HER2-positive disease might be practice changing. It offers an appealing, perhaps therapeutic, option for patients with ER-positive, HER2-positive metastatic disease. Now you can consider targeting HER2 and ER with an oral-only regimen.
Track 3
![]() DR LOVE: So at this point, how do you think through treatment for a
patient with ER-positive, HER2-positive metastatic disease who has not
received prior anti-HER2 therapy?
DR LOVE: So at this point, how do you think through treatment for a
patient with ER-positive, HER2-positive metastatic disease who has not
received prior anti-HER2 therapy?
![]() DR PEGRAM: Disease that is naïve to HER2-targeted agents responds well to
either trastuzumab or lapatinib. You can present the pros and cons of each to
the patients and allow them to participate in the decision. It depends on IV
access, cardiac history and so on. Either agent is acceptable.
DR PEGRAM: Disease that is naïve to HER2-targeted agents responds well to
either trastuzumab or lapatinib. You can present the pros and cons of each to
the patients and allow them to participate in the decision. It depends on IV
access, cardiac history and so on. Either agent is acceptable.
Another option, based on data presented by Joyce O’Shaughnessy at ASCO 2008, is the combination of lapatinib and trastuzumab (O’Shaughnessy 2008).
I was a coauthor of the recent Phase I trial of that regimen, and we recorded some remarkable anecdotal activity (Storniolo 2008). Joyce O’Shaughnessy’s randomized trial clearly shows that the combination is efficacious (O’Shaughnessy 2008).
![]() DR LOVE: What about the use of endocrine therapy alone and postponing the
use of anti-HER2 agents as first-line therapy for metastatic disease?
DR LOVE: What about the use of endocrine therapy alone and postponing the
use of anti-HER2 agents as first-line therapy for metastatic disease?
![]() DR PEGRAM: The results in a population with HER2-positive disease are
disappointing, but a few percent will achieve long periods of progression-free
survival with endocrine manipulation alone.
DR PEGRAM: The results in a population with HER2-positive disease are
disappointing, but a few percent will achieve long periods of progression-free
survival with endocrine manipulation alone.
It’s certainly on the table for discussion with patients, and many view it as a viable option. You must follow those patients closely, however, because their disease-progression rates can be extreme in the case of HER2-positive disease.
Track 5
![]() DR LOVE: What were your thoughts on the German trial presented at
ASCO 2008 by von Minckwitz?
DR LOVE: What were your thoughts on the German trial presented at
ASCO 2008 by von Minckwitz?
![]() DR PEGRAM: Whether any benefit exists in the continuation of trastuzumab after
the first disease progression has long been debated. The cooperative groups in the
United States had attempted to conduct a randomized trial twice in the past.
DR PEGRAM: Whether any benefit exists in the continuation of trastuzumab after
the first disease progression has long been debated. The cooperative groups in the
United States had attempted to conduct a randomized trial twice in the past.
They failed because of lack of accrual because the bias in the United States was to keep using trastuzumab for patients with HER2-positive disease through multiple lines of disease progression. As a consequence of that bias, it was difficult to convince referring doctors, investigators and patients to randomize to a nontrastuzumab-containing arm.
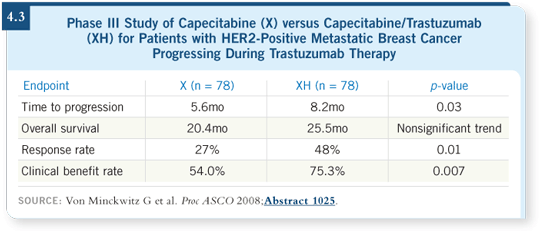
In Europe this was less problematic, and they accomplished the study, which von Minckwitz reported. Sure enough, continuation of trastuzumab with capecitabine was statistically superior compared to capecitabine and discontinuation of trastuzumab in terms of time to disease progression in the salvage second-line setting (Von Minckwitz 2008; [4.3]). This trial supports the overarching concept of prolonged HER2 perturbation in metastatic disease.
Tracks 14-15
![]() DR LOVE: What are your thoughts on the Finnish study ( Joensuu 2008)
that was presented at San Antonio, which is similar to the major, unreported
adjuvant clinical trial Joyce O’Shaughnessy and US Oncology are conducting
that compares AC
DR LOVE: What are your thoughts on the Finnish study ( Joensuu 2008)
that was presented at San Antonio, which is similar to the major, unreported
adjuvant clinical trial Joyce O’Shaughnessy and US Oncology are conducting
that compares AC ![]() docetaxel to AC
docetaxel to AC ![]() docetaxel/capecitabine?
docetaxel/capecitabine?
![]() DR PEGRAM: The Finnish trial was interesting and had a similar basis to
Joyce’s study, specifically the concept of upregulating thymidine phosphorylase,
which is the final step in conversion of the capecitabine prodrug analog
in its various metabolites into 5-f luoropyrimidine, intratumorally. Docetaxel
can upregulate thymidine phosphorylase, and that was the rationale for
combining capecitabine with docetaxel.
DR PEGRAM: The Finnish trial was interesting and had a similar basis to
Joyce’s study, specifically the concept of upregulating thymidine phosphorylase,
which is the final step in conversion of the capecitabine prodrug analog
in its various metabolites into 5-f luoropyrimidine, intratumorally. Docetaxel
can upregulate thymidine phosphorylase, and that was the rationale for
combining capecitabine with docetaxel.
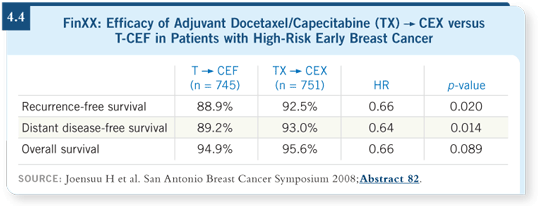
The FinXX trial randomly assigned approximately 1,500 patients with early-stage breast cancer to an interesting standard arm: Docetaxel at 80 mg/m2 for three cycles followed by CEF versus docetaxel at 60 mg/m2 with capecitabine at 900 mg/m2 BID followed by CE with capecitabine at 900 mg/m2 (CEX).
They demonstrated a statistically significant improvement in distant disease-free survival in favor of the capecitabine arm, with a hazard ratio of 0.64 (Joensuu 2008; [4.4]). It’s an intriguing observation and supports the concept of integrating capecitabine into the adjuvant setting. On the other hand, the control arm is not one that we use, so I’m uncertain how to incorporate this information into my clinical practice. If another randomized Phase III trial, such as the US Oncology study, showed similar efficacy, then it would probably move capecitabine into the limelight.
![]() DR LOVE: When I spoke to Joyce, we were questioning whether this could
become another “TC” regimen with capecitabine/docetaxel as opposed to
cyclophosphamide/docetaxel.
DR LOVE: When I spoke to Joyce, we were questioning whether this could
become another “TC” regimen with capecitabine/docetaxel as opposed to
cyclophosphamide/docetaxel.
![]() DR PEGRAM: Or DCF, with docetaxel, cyclophosphamide and a fluoropyrimidine,
which is similar to the regimen used in gastric cancer or head and neck
cancer.
DR PEGRAM: Or DCF, with docetaxel, cyclophosphamide and a fluoropyrimidine,
which is similar to the regimen used in gastric cancer or head and neck
cancer.
EDITOR
Neil Love, MD
Harold J Burstein, MD, PhD
- Select publications
Jack Cuzick, PhD
- Select publications
Howard A Burris III, MD
- Select publications
Mark D Pegram, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity