
 |
||||||||

| Tracks 1-9 | ||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you review the AI meta-analyses presented by Jim Ingle at the 2008 San Antonio Breast Cancer Symposium?
DR LOVE: Would you review the AI meta-analyses presented by Jim Ingle at the 2008 San Antonio Breast Cancer Symposium?
![]() PROF CUZICK: We evaluated two separate cohorts: patients receiving adjuvant
treatment with an up-front aromatase inhibitor versus tamoxifen and patients
receiving a switching strategy of tamoxifen for approximately two years
followed by a further three years of tamoxifen or an aromatase inhibitor
(Ingle 2008).
PROF CUZICK: We evaluated two separate cohorts: patients receiving adjuvant
treatment with an up-front aromatase inhibitor versus tamoxifen and patients
receiving a switching strategy of tamoxifen for approximately two years
followed by a further three years of tamoxifen or an aromatase inhibitor
(Ingle 2008).
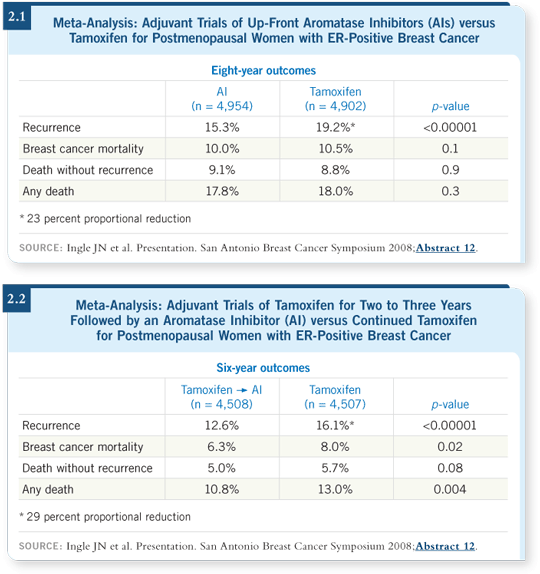
We found no surprises. Up-front therapy with an aromatase inhibitor versus tamoxifen reduced the rate of relapse by approximately 23 percent. However, no difference in breast cancer survival was apparent in the up-front therapy trials (Ingle 2008; [2.1]). The challenge was that the switching trials could not be directly compared to the up-front trials because they included different patient populations. The results of the switching studies were generally similar to those of the up-front studies in terms of recurrence. However, the switching studies have also shown a reduction in overall mortality (Ingle 2008; [2.2]).
Tracks 2-3
![]() DR LOVE: Would you discuss the updated results of the BIG 1-98 study
presented at the SABCS meeting?
DR LOVE: Would you discuss the updated results of the BIG 1-98 study
presented at the SABCS meeting?
![]() PROF CUZICK: The update comparing up-front letrozole to tamoxifen was
difficult to interpret because, in response to early results from ATAC and
other trials, the patients on the tamoxifen arm were unblinded and one fourth
of patients chose to switch to letrozole (Mouridsen 2008).
PROF CUZICK: The update comparing up-front letrozole to tamoxifen was
difficult to interpret because, in response to early results from ATAC and
other trials, the patients on the tamoxifen arm were unblinded and one fourth
of patients chose to switch to letrozole (Mouridsen 2008).
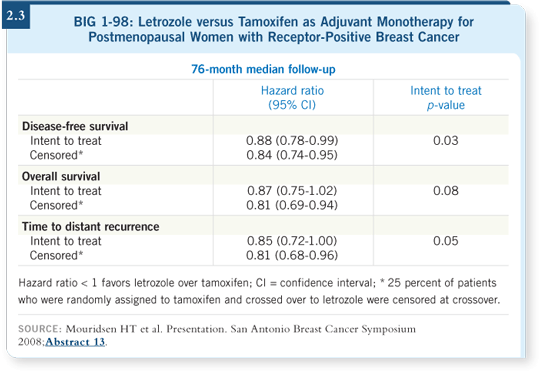
The conventional analysis for all patients as randomly assigned (intent to treat) showed a nonsignificant trend toward better overall survival with up-front letrozole. The alternate analysis, which censored patients when they crossed over from tamoxifen to letrozole, showed a significant effect of letrozole on overall survival (Mouridsen 2008; [2.3]).
![]() DR LOVE: What were the results from the sequencing aspect of BIG 1-98?
DR LOVE: What were the results from the sequencing aspect of BIG 1-98?
![]() PROF CUZICK: The trial enrolled approximately 1,500 patients per arm (Mouridsen
2008). The differences between the sequential and up-front use of an
aromatase inhibitor are smaller than the differences between letrozole and
tamoxifen. So we need trials that are bigger than any of the individual trials, and
BIG 1-98 was smaller. It was clear that no differences would be evident.
PROF CUZICK: The trial enrolled approximately 1,500 patients per arm (Mouridsen
2008). The differences between the sequential and up-front use of an
aromatase inhibitor are smaller than the differences between letrozole and
tamoxifen. So we need trials that are bigger than any of the individual trials, and
BIG 1-98 was smaller. It was clear that no differences would be evident.
Tracks 5, 7
![]() DR LOVE: Would you discuss the paper you recently published in The
Lancet Oncology evaluating endocrine therapy?
DR LOVE: Would you discuss the paper you recently published in The
Lancet Oncology evaluating endocrine therapy?
![]() PROF CUZICK: We evaluated patients who reported endocrine symptoms
— such as hot flashes, night sweats or arthralgias — during the first follow-up
visit at three months in the ATAC trial.
PROF CUZICK: We evaluated patients who reported endocrine symptoms
— such as hot flashes, night sweats or arthralgias — during the first follow-up
visit at three months in the ATAC trial.
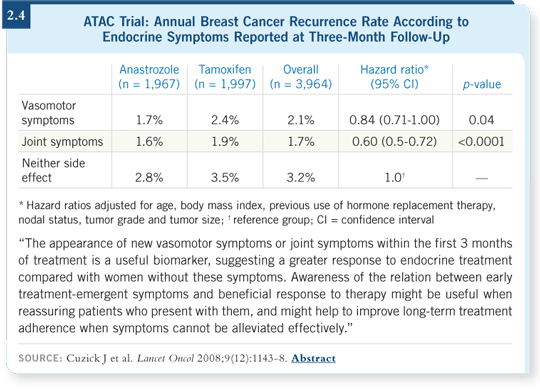
Although more arthralgias were reported in the anastrozole arm and more hot flashes were reported in the tamoxifen arm, the overall numbers of patients reporting symptoms were about the same. Approximately 50 percent of the women in each arm had something to report at three months (Cuzick 2008).
Then we evaluated recurrences subsequent to that visit. The striking observation was that in both treatment arms, patients with symptoms fared substantially better than patients without symptoms. The size of the effect was larger than the difference between tamoxifen and anastrozole (Cuzick 2008; [2.4]).
The first value of these results is that they will encourage women who have mild to moderate symptoms to recognize that this is an indicator that the drug is doing what it’s meant to do. So we hope it will encourage compliance, which is a crucial issue in the use of these drugs.
EDITOR
Neil Love, MD
Harold J Burstein, MD, PhD
- Select publications
Jack Cuzick, PhD
- Select publications
Howard A Burris III, MD
- Select publications
Mark D Pegram, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity