
 |
||||||||

| Tracks 1-10 | ||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-2
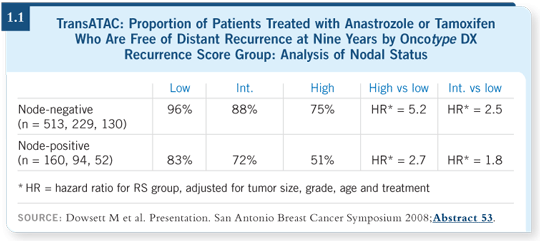
![]() DR LOVE: Would you discuss the TransATAC analysis data presented at the 2008 San Antonio Breast Cancer Symposium (1.1)?
DR LOVE: Would you discuss the TransATAC analysis data presented at the 2008 San Antonio Breast Cancer Symposium (1.1)?
![]() DR BURSTEIN: The ATAC investigators in collaboration with Genomic
Health analyzed data from a subset of approximately 1,200 patients who
received endocrine therapy only. This was not a randomly selected subset of
patients. However, it was a large, representative subset from the ATAC study.
They reported that the trends, in terms of using the Oncotype DX Recurrence
Score® to predict the likelihood of distant metastatic disease through nine years
of follow-up, were similar with tamoxifen and anastrozole (Dowsett 2008).
Dr Burstein is Assistant Professor of Medicine at the
Harvard Medical School Breast Oncology Center at
Dana-Farber Cancer Institute in Boston, Massachusetts.
DR BURSTEIN: The ATAC investigators in collaboration with Genomic
Health analyzed data from a subset of approximately 1,200 patients who
received endocrine therapy only. This was not a randomly selected subset of
patients. However, it was a large, representative subset from the ATAC study.
They reported that the trends, in terms of using the Oncotype DX Recurrence
Score® to predict the likelihood of distant metastatic disease through nine years
of follow-up, were similar with tamoxifen and anastrozole (Dowsett 2008).
Dr Burstein is Assistant Professor of Medicine at the
Harvard Medical School Breast Oncology Center at
Dana-Farber Cancer Institute in Boston, Massachusetts.
That is to say, you can utilize the Oncotype DX assay to determine the risk estimates for patients being treated with aromatase inhibitors and for patients being treated with tamoxifen.
![]() DR LOVE: What about the issue of quantitative assessment of ER and HER2,
which is being reported in the Oncotype DX assay?
DR LOVE: What about the issue of quantitative assessment of ER and HER2,
which is being reported in the Oncotype DX assay?
![]() DR BURSTEIN: Quantitative HER2 testing has not yet yielded subsets of
patients who should or should not receive HER2-directed therapy. For ER
testing, we have known for 40 years that more ER in a tumor translates into
increased sensitivity to endocrine therapy.
DR BURSTEIN: Quantitative HER2 testing has not yet yielded subsets of
patients who should or should not receive HER2-directed therapy. For ER
testing, we have known for 40 years that more ER in a tumor translates into
increased sensitivity to endocrine therapy.
In Giuseppe Viale’s papers from the BIG 1-98 study, patients whose tumors were strongly ER-positive fared well with either tamoxifen or letrozole (Viale 2007), whereas those who had tumors with lower levels of ER and/or high Ki-67 did not fare quite as well (Viale 2008). Similarly, patients with ER-positive, HER2-positive disease demonstrated poorer outcomes, but patients treated with letrozole fared better than those treated with tamoxifen (Rasmussen 2008). So these are important biomarkers and the Oncotype DX is built around all of them, which is an appealing aspect of this assay since it resonates with all of this other biomarker literature.
EDITOR
Neil Love, MD
Harold J Burstein, MD, PhD
- Select publications
Jack Cuzick, PhD
- Select publications
Howard A Burris III, MD
- Select publications
Mark D Pegram, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity