
 |
||||||||

| Tracks 1-12 | ||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 4-5
![]() DR LOVE: What is your opinion of the data from the TransATAC study presented at San Antonio on Oncotype DX?
DR LOVE: What is your opinion of the data from the TransATAC study presented at San Antonio on Oncotype DX?
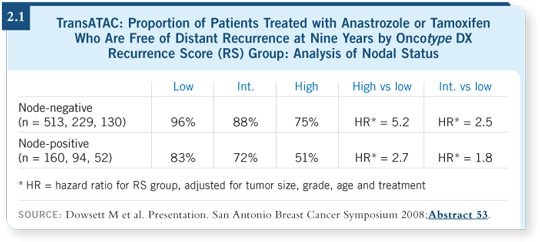
![]() DR JONES: The Oncotype DX Recurrence Score proved to be an independent predictor of the risk of distant recurrence in postmenopausal patients with ER-positive, node-negative or node-positive breast cancer treated with either tamoxifen or anastrozole (Dowsett 2008; [2.1]).
DR JONES: The Oncotype DX Recurrence Score proved to be an independent predictor of the risk of distant recurrence in postmenopausal patients with ER-positive, node-negative or node-positive breast cancer treated with either tamoxifen or anastrozole (Dowsett 2008; [2.1]).
The Oncotype DX data have been consistent in a variety of settings, and we haven’t seen any surprises. The assay was consistent in one series with node-positive patients, in which they received tamoxifen or chemotherapy in combination with tamoxifen (Albain 2007). It’s been consistent in all the tamoxifen series. Now it’s consistent in the ATAC series. That’s why I believe this assay is so far along.
This assay humbles us a bit. I consider myself to be an experienced breast oncologist. I’ve done this for 35 years. I think I can tell who needs chemotherapy and who doesn’t. That’s my arrogance, but biology and these tests are starting to trump my personal opinion.
Tracks 10, 12
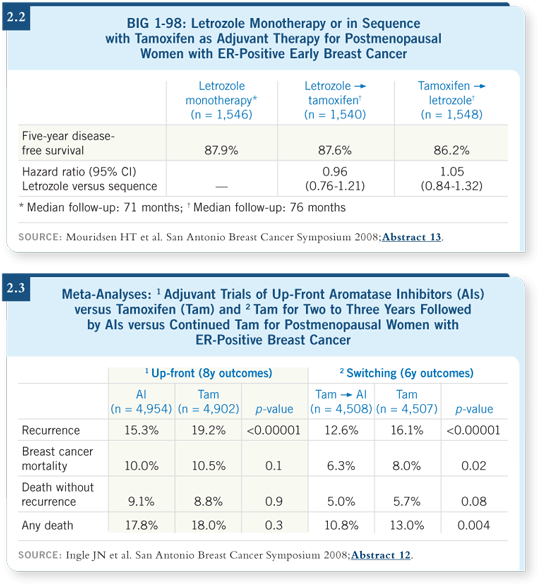
![]() DR LOVE: In addition to a presentation with the sequencing data for BIG
1-98 (Mouridsen 2008; [2.2]), two other important data sets for adjuvant
endocrine therapy were presented on the first morning of the San
Antonio meeting (Ingle 2008; Jones 2008). Would you summarize your
impressions of the data?
DR LOVE: In addition to a presentation with the sequencing data for BIG
1-98 (Mouridsen 2008; [2.2]), two other important data sets for adjuvant
endocrine therapy were presented on the first morning of the San
Antonio meeting (Ingle 2008; Jones 2008). Would you summarize your
impressions of the data?
![]() DR JONES: First, Jim Ingle presented a meta-analysis examining two cohorts
of patients who were treated with different adjuvant endocrine approaches
(Ingle 2008; [2.3]).
DR JONES: First, Jim Ingle presented a meta-analysis examining two cohorts
of patients who were treated with different adjuvant endocrine approaches
(Ingle 2008; [2.3]).
The first cohort evaluated up-front aromatase inhibitors versus tamoxifen, comprised predominantly of patients from the ATAC and BIG 1-98 studies. A definite reduction in recurrence rates was evident with the up-front aromatase inhibitors, but no difference in overall survival was noted. The second cohort evaluated switching from two to three years of adjuvant tamoxifen to an aromatase inhibitor. This analysis also demonstrated a reduction in recurrence rates for the switching strategy, but more importantly, an improvement in overall survival.
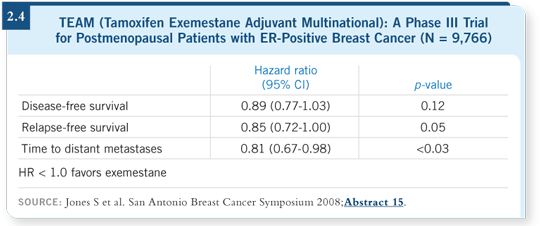
![]() DR LOVE: I think there has been some confusion about trying to compare
the two strategies, because the switching studies focused on patients who completed two to three years of tamoxifen and did not experience relapse.
What was shown in your presentation of the TEAM study comparing up-front
tamoxifen to exemestane ( Jones 2008; [2.4])?
DR LOVE: I think there has been some confusion about trying to compare
the two strategies, because the switching studies focused on patients who completed two to three years of tamoxifen and did not experience relapse.
What was shown in your presentation of the TEAM study comparing up-front
tamoxifen to exemestane ( Jones 2008; [2.4])?
![]() DR JONES: A message came through loud and clear the first morning of the
San Antonio meeting, and I was pleased to be the third one to present that
message. The TEAM study has been the missing link in that it was the first
study with an up-front comparison of tamoxifen to an aromatase inactivator,
which has a different mechanism of action than the other nonsteroidal aromatase
inhibitors.
DR JONES: A message came through loud and clear the first morning of the
San Antonio meeting, and I was pleased to be the third one to present that
message. The TEAM study has been the missing link in that it was the first
study with an up-front comparison of tamoxifen to an aromatase inactivator,
which has a different mechanism of action than the other nonsteroidal aromatase
inhibitors.
In TEAM, the intent-to-treat hazard ratio was 0.89, or an 11 percent reduction in recurrence. However, when we analyzed the study according to patients who were still receiving the treatment to which they were randomly assigned, the HR was 0.83, or a 17 percent reduction.
EDITOR
Neil Love, MD
Edith A Perez, MD
- Select publications
Stephen E Jones, MD
- Select publications
Sandra M Swain, MD
- Select publications
Ian E Krop, MD, PhD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity