
 |
||||||||

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you discuss the recently opened NSABP/CIRG collaborative
BETH adjuvant trial for patients with HER2-positive breast
cancer?
DR LOVE: Would you discuss the recently opened NSABP/CIRG collaborative
BETH adjuvant trial for patients with HER2-positive breast
cancer?
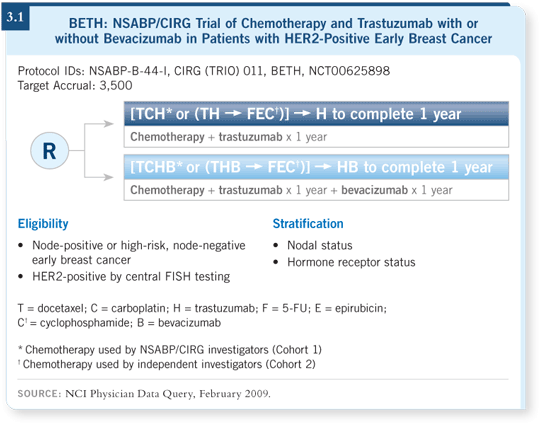
![]() DR SWAIN: The NSABP has joined with the CIRG to conduct a large
adjuvant study for patients with HER2-positive disease. The BETH study is
based on Dennis Slamon and Mark Pegram’s preclinical data, the Phase I and Phase II study combining trastuzumab with bevacizumab (Pegram 2006) and
the BCIRG 006 study using docetaxel, carboplatin and trastuzumab (TCH;
[Slamon 2006]).
DR SWAIN: The NSABP has joined with the CIRG to conduct a large
adjuvant study for patients with HER2-positive disease. The BETH study is
based on Dennis Slamon and Mark Pegram’s preclinical data, the Phase I and Phase II study combining trastuzumab with bevacizumab (Pegram 2006) and
the BCIRG 006 study using docetaxel, carboplatin and trastuzumab (TCH;
[Slamon 2006]).
The BETH study is open to almost every patient with HER2-positive disease, even those with node-negative disease. Patients will be randomly assigned to TCH with or without bevacizumab (3.1).
![]() DR LOVE: What’s the biologic and clinical rationale for this trial?
DR LOVE: What’s the biologic and clinical rationale for this trial?
![]() DR SWAIN: Dennis Slamon examined approximately 600 tumors and showed
that those that were HER2-positive and had a high VEGF expression had a
worse prognosis (Konecny 2004). After showing synergy with the combination
of trastuzumab and bevacizumab in preclinical studies, he evaluated the
combination in a Phase II study of HER2-positive advanced breast cancer
and the overall response rate was approximately 50 percent (Pegram 2006).
So it’s nicely going from the bench to the bedside, as Dr Slamon did with the
BCIRG 006 study (Slamon 2006).
DR SWAIN: Dennis Slamon examined approximately 600 tumors and showed
that those that were HER2-positive and had a high VEGF expression had a
worse prognosis (Konecny 2004). After showing synergy with the combination
of trastuzumab and bevacizumab in preclinical studies, he evaluated the
combination in a Phase II study of HER2-positive advanced breast cancer
and the overall response rate was approximately 50 percent (Pegram 2006).
So it’s nicely going from the bench to the bedside, as Dr Slamon did with the
BCIRG 006 study (Slamon 2006).
![]() DR LOVE: What do we know about the safety of combining trastuzumab and
bevacizumab?
DR LOVE: What do we know about the safety of combining trastuzumab and
bevacizumab?
![]() DR SWAIN: In the Phase II study 13 patients had a decrease in ejection
fraction (EF), with one severe heart failure. Most of these decreases in EF
were Grade I and were not something you would act on. However, hypertension
is a known toxicity of bevacizumab, and with a big afterload we may see more cardiac toxicity in combination with trastuzumab. Denise Yardley in the
Sarah Cannon Group presented at San Antonio three different parallel studies
evaluating bevacizumab in combination with docetaxel regimens — TAC,
AC
DR SWAIN: In the Phase II study 13 patients had a decrease in ejection
fraction (EF), with one severe heart failure. Most of these decreases in EF
were Grade I and were not something you would act on. However, hypertension
is a known toxicity of bevacizumab, and with a big afterload we may see more cardiac toxicity in combination with trastuzumab. Denise Yardley in the
Sarah Cannon Group presented at San Antonio three different parallel studies
evaluating bevacizumab in combination with docetaxel regimens — TAC,
AC ![]() T or TCH — to study the cardiac toxicity (Yardley 2008; [3.2]). One
heart failure occurred in the study of TCH with bevacizumab, three in the
TAC group and three in patients who received AC
T or TCH — to study the cardiac toxicity (Yardley 2008; [3.2]). One
heart failure occurred in the study of TCH with bevacizumab, three in the
TAC group and three in patients who received AC ![]() T. In the BETH study,
we are carefully monitoring EF and are conducting a cardiac analysis of several
hundred patients, similar to the B-31 study and the N9831 study, to make sure
no excessive cardiac toxicity is incurred.
T. In the BETH study,
we are carefully monitoring EF and are conducting a cardiac analysis of several
hundred patients, similar to the B-31 study and the N9831 study, to make sure
no excessive cardiac toxicity is incurred.

Track 14
![]() DR LOVE: Would you discuss the FinXX study evaluating the addition of
capecitabine to a taxane/anthracycline base regimen?
DR LOVE: Would you discuss the FinXX study evaluating the addition of
capecitabine to a taxane/anthracycline base regimen?
![]() DR SWAIN: In this trial, patients were randomly assigned to treatment with
three cycles of docetaxel (T)
DR SWAIN: In this trial, patients were randomly assigned to treatment with
three cycles of docetaxel (T) ![]() cyclophosphamide, epirubicin and
5-fluorouracil (CEF) or docetaxel and capecitabine (XT)
cyclophosphamide, epirubicin and
5-fluorouracil (CEF) or docetaxel and capecitabine (XT) ![]() cyclophosphamide,
epirubicin and capecitabine (CEX; [Joensuu 2008]). It included 1,500
patients with node-positive disease and node-negative tumors.
cyclophosphamide,
epirubicin and capecitabine (CEX; [Joensuu 2008]). It included 1,500
patients with node-positive disease and node-negative tumors.
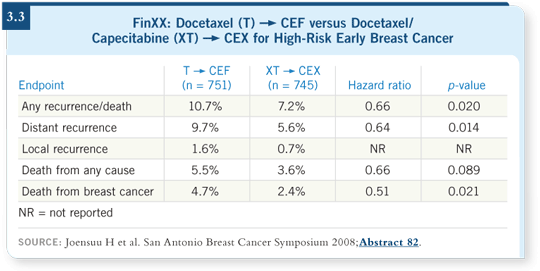
The results were striking (3.3). Recurrence-free survival was significantly
better in the XT ![]() CEX group. It’s definitely a positive trial. They found 80
events in the T
CEX group. It’s definitely a positive trial. They found 80
events in the T ![]() CEF arm and 54 events in the capecitabine arm. You can’t
argue with it. The distant events were 72 versus 42. It appeared to be active.
CEF arm and 54 events in the capecitabine arm. You can’t
argue with it. The distant events were 72 versus 42. It appeared to be active.
The design of the FinXX trial was excellent. It was based on previous studies
using docetaxel/capecitabine (O’Shaughnessy 2002), so it makes sense that it’s
beneficial, but it’s not enough for me to change my treatment approach now.
However, it makes me think about it, and I am anxious to see data from Joyce
O’Shaughnessy and the US Oncology trial evaluating AC![]() T versus AC
T versus AC![]() XT.
XT.
The other point is that evaluating adverse events is where we’re headed with
the chemotherapy trials. We want the fewest adverse events possible. In the
FinXX trial, the TX ![]() CEX arm resulted in less toxicity. Of all the different
toxicities, the febrile neutropenia and the myalgias were more prominent
in the group that did not receive capecitabine. So I believe that it could be
something people will want to use in the future.
CEX arm resulted in less toxicity. Of all the different
toxicities, the febrile neutropenia and the myalgias were more prominent
in the group that did not receive capecitabine. So I believe that it could be
something people will want to use in the future.
EDITOR
Neil Love, MD
Edith A Perez, MD
- Select publications
Stephen E Jones, MD
- Select publications
Sandra M Swain, MD
- Select publications
Ian E Krop, MD, PhD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity