
 |
||||||||

| Tracks 1-10 | ||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you discuss the recent editorial you published on the
evolution of clinical research data with trastuzumab (Krop 2009)?
DR LOVE: Would you discuss the recent editorial you published on the
evolution of clinical research data with trastuzumab (Krop 2009)?
![]() DR KROP: It has been approximately 10 years since trastuzumab was
approved, and we still have a number of unanswered questions that this editorial
examined (Krop 2009). First, what is this concept of resistance to trastuzumab?
Second, when does the benefit of trastuzumab stop? This is difficult
to define because trastuzumab is not typically administered alone.
DR KROP: It has been approximately 10 years since trastuzumab was
approved, and we still have a number of unanswered questions that this editorial
examined (Krop 2009). First, what is this concept of resistance to trastuzumab?
Second, when does the benefit of trastuzumab stop? This is difficult
to define because trastuzumab is not typically administered alone.
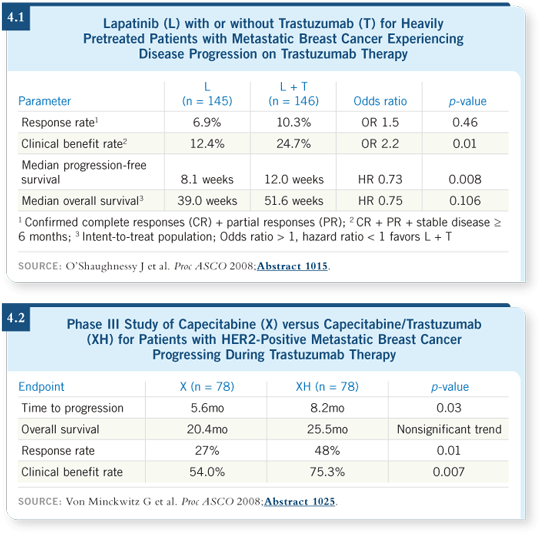
Two presentations at ASCO 2008 may have provided some clarification to this editorial. In a presentation by Joyce O’Shaughnessy evaluating patients whose disease had progressed on multiple lines of trastuzumab-based therapy, patients were randomly assigned to lapatinib alone or in combination with trastuzumab. Despite the fact that the patients’ disease had progressed not on only one but, in most cases, multiple lines of trastuzumab-based therapy, a significant benefit was seen from continuing trastuzumab with the addition of lapatinib (O’Shaughnessy 2008; [4.1]). Also, a German study by von Minckwitz reported a benefit to continuing trastuzumab in patients treated with capecitabine alone or in combination with trastuzumab after disease progression on trastuzumab therapy (von Minckwitz 2008; [4.2]).
I believe that these two studies indicate that, at least for a significant number of patients, disease progression on a trastuzumab-based regimen was probably due in part to resistance to the agent with which trastuzumab was combined rather than to trastuzumab itself.
Track 5
![]() DR LOVE: Would you discuss the mechanism of action with T-DM1 and
the results you presented at the last San Antonio meeting?
DR LOVE: Would you discuss the mechanism of action with T-DM1 and
the results you presented at the last San Antonio meeting?
![]() DR KROP: T-DM1 is the monoclonal antibody trastuzumab chemically
linked to a cytotoxic agent, in this case the antimicrotubule agent DM1. The antibody specifically targets the cytotoxic agent to the tumor cell. So the idea
is that you’re able to deliver high amounts of your cytotoxic agent directly to
the tumor cell while sparing normal tissue from toxicity.
DR KROP: T-DM1 is the monoclonal antibody trastuzumab chemically
linked to a cytotoxic agent, in this case the antimicrotubule agent DM1. The antibody specifically targets the cytotoxic agent to the tumor cell. So the idea
is that you’re able to deliver high amounts of your cytotoxic agent directly to
the tumor cell while sparing normal tissue from toxicity.
The Phase I data found encouraging levels of activity — despite the fact that patients had been heavily pretreated — with objective, confirmed response rates in the 40 to 50 percent range (Krop 2008). Another aspect of this drug is how well tolerated it is. The significant toxicities are transient thrombocytopenia and transaminase elevation, but at the maximum tolerated dose, both of those problems are clinically unapparent.
Track 9
![]() DR LOVE: Can you discuss the work conducted by your colleague
Nancy Lin, evaluating lapatinib for the treatment of HER2-positive
CNS metastases?
DR LOVE: Can you discuss the work conducted by your colleague
Nancy Lin, evaluating lapatinib for the treatment of HER2-positive
CNS metastases?
![]() DR KROP: CNS metastases develop in approximately 30 to 40 percent of
patients with advanced HER2-positive breast cancer (Lin 2008). It’s possible
that by using a small molecule such as lapatinib we may be able to have an
effect on this site of disease.
DR KROP: CNS metastases develop in approximately 30 to 40 percent of
patients with advanced HER2-positive breast cancer (Lin 2008). It’s possible
that by using a small molecule such as lapatinib we may be able to have an
effect on this site of disease.
Nancy Lin and colleagues at Dana-Farber initiated a small study of single-agent lapatinib in patients who had CNS metastases from HER2-positive breast cancer that progressed despite palliative radiation therapy, so we do not have many options for those patients (Lin 2008). She observed a small but significant rate of CNS responses. The study was expanded to combine lapatinib with capecitabine in these patients, and again, a small but significant number of patients benefited (Lin 2009). So currently she’s evaluating combining other chemotherapeutic agents, including epothilones, with lapatinib for patients with CNS metastases.
EDITOR
Neil Love, MD
Edith A Perez, MD
- Select publications
Stephen E Jones, MD
- Select publications
Sandra M Swain, MD
- Select publications
Ian E Krop, MD, PhD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity