
 |
||||||||

| Tracks 1-19 | ||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 6-7
![]() DR LOVE: Would you summarize where we are currently in terms of clinical research data on bevacizumab for metastatic breast cancer?
DR LOVE: Would you summarize where we are currently in terms of clinical research data on bevacizumab for metastatic breast cancer?
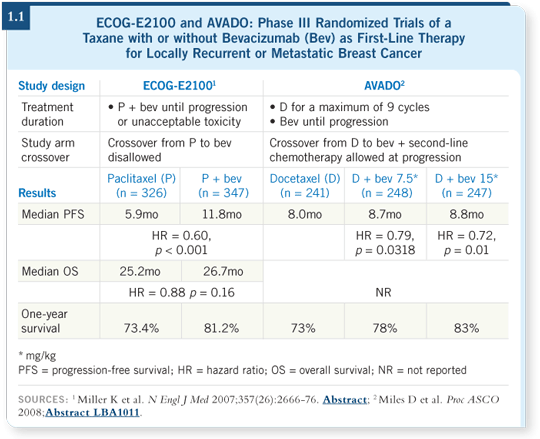
![]() DR PEREZ: ECOG-E2100 demonstrated a dramatic improvement in progression-free survival with weekly paclitaxel/bevacizumab as first-line therapy, but no statistical difference in overall survival was seen (Miller 2007; [1.1]). The two agents were continued until disease progression or prohibitive toxic effects occurred.
DR PEREZ: ECOG-E2100 demonstrated a dramatic improvement in progression-free survival with weekly paclitaxel/bevacizumab as first-line therapy, but no statistical difference in overall survival was seen (Miller 2007; [1.1]). The two agents were continued until disease progression or prohibitive toxic effects occurred.
In the AVADO trial, a statistically significant improvement in median progression-free survival was found for docetaxel/bevacizumab in the first-line setting, but the difference was less than one month (Miles 2008; [1.1]). We can say that the AVADO trial corroborated ECOG-E2100, but it didn’t corroborate it to the degree I would have liked.
Potential explanations are related to the differences between the two trials. In the AVADO trial, the patients received up to nine doses of docetaxel. At the beginning, the patients received docetaxel/bevacizumab, and then the physicians had the option of discontinuing docetaxel and continuing bevacizumab as a single agent (Miles 2008).
![]() DR LOVE: Another potential issue is related to the choice and schedule of
taxanes — weekly paclitaxel versus every three-week docetaxel — and their
effectiveness as anti-angiogenic agents.
DR LOVE: Another potential issue is related to the choice and schedule of
taxanes — weekly paclitaxel versus every three-week docetaxel — and their
effectiveness as anti-angiogenic agents.
![]() DR PEREZ: That’s possible, because both taxanes have anti-angiogenic
properties, but a weekly schedule of administration may be more effective.
That’s one of the reasons why RIBBON 1 will be so interesting.
DR PEREZ: That’s possible, because both taxanes have anti-angiogenic
properties, but a weekly schedule of administration may be more effective.
That’s one of the reasons why RIBBON 1 will be so interesting.
Track 5
![]() DR LOVE: Can you comment on the Finnish study that was presented
at San Antonio, which added capecitabine to docetaxel followed by an
anthracycline in the adjuvant setting (Joensuu 2008)?
DR LOVE: Can you comment on the Finnish study that was presented
at San Antonio, which added capecitabine to docetaxel followed by an
anthracycline in the adjuvant setting (Joensuu 2008)?
![]() DR PEREZ: This was a provocative trial. The follow-up is short, but the
study demonstrated that the addition of capecitabine led to an improvement
in disease-free survival (Joensuu 2008; [3.3]), which is consistent
with the docetaxel/capecitabine data reported in metastatic breast cancer
(O’Shaughnessy 2002).
DR PEREZ: This was a provocative trial. The follow-up is short, but the
study demonstrated that the addition of capecitabine led to an improvement
in disease-free survival (Joensuu 2008; [3.3]), which is consistent
with the docetaxel/capecitabine data reported in metastatic breast cancer
(O’Shaughnessy 2002).
The investigators diminished the dose of docetaxel to 80 mg/m2 instead of 100 mg/m2, and they also reduced the dose of capecitabine to 900 mg/m2 twice per day. This is a good regimen, and it will be interesting to see longer follow-up of that trial.
Track 4
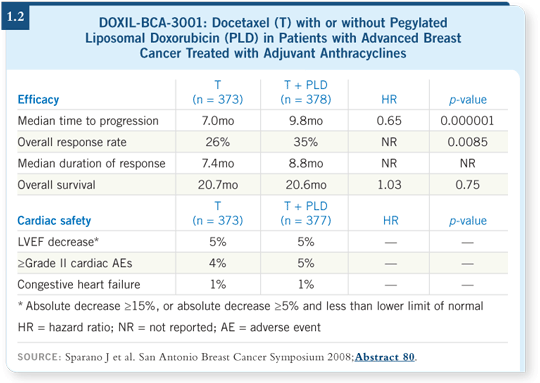
![]() DR LOVE: Joe Sparano presented data at San Antonio from a study of
liposomal doxorubicin with docetaxel in patients with advanced breast
cancer (Sparano 2008; [1.2]). What are your thoughts about the role of
these agents in breast cancer management?
DR LOVE: Joe Sparano presented data at San Antonio from a study of
liposomal doxorubicin with docetaxel in patients with advanced breast
cancer (Sparano 2008; [1.2]). What are your thoughts about the role of
these agents in breast cancer management?
![]() DR PEREZ: Liposomal anthracyclines are important drugs. When the original
study was conducted comparing liposomal anthracyclines to standard doxorubicin
in a large number of patients, they were able to demonstrate that patients
could receive more anthracycline with the pegylated liposomal encapsulation
of the drug (O’Brien 2004).
DR PEREZ: Liposomal anthracyclines are important drugs. When the original
study was conducted comparing liposomal anthracyclines to standard doxorubicin
in a large number of patients, they were able to demonstrate that patients
could receive more anthracycline with the pegylated liposomal encapsulation
of the drug (O’Brien 2004).
However, it was difficult to demonstrate statistically significant improvements in survival or disease-free survival. Currently, an ongoing randomized trial for patients with HER2-positive breast cancer is evaluating paclitaxel/trastuzumab versus paclitaxel/trastuzumab/liposomal doxorubicin.
This strategy is based on fascinating Phase III data presented by Jose Baselga and colleagues in the neoadjuvant setting, in which a huge response rate to triplet therapy was demonstrated with essentially no cardiac toxicity when the anthracycline was administered concurrently with trastuzumab (Gianni 2008).
EDITOR
Neil Love, MD
Edith A Perez, MD
- Select publications
Stephen E Jones, MD
- Select publications
Sandra M Swain, MD
- Select publications
Ian E Krop, MD, PhD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity