|

| Tracks 1-19 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Trial E2100: Bevacizumab with paclitaxel as first-line treatment of metastatic disease |
| Track 3 |
Progression-free survival as an endpoint in metastatic disease trials |
| Track 4 |
Mechanism of action of bevacizumab |
| Track 5 |
Integration of bevacizumab into treatment in the metastatic setting |
| Track 6 |
Clinical use of nanoparticle albumin-bound (nab) paclitaxel |
| Track 7 |
Contraindications for utilization of bevacizumab |
| Track 8 |
Future directions in clinical trials of bevacizumab |
| Track 9 |
Impact of adjuvant trastuzumab data on future trials of adjuvant chemotherapy |
|
| Track 10 |
Combined analysis of NSABP-B-31/NCCTG-N9831 trials of adjuvant trastuzumab |
| Track 11 |
Results of the HERA adjuvant trastuzumab trial |
| Track 12 |
Cardiac toxicity data from clinical trials of adjuvant trastuzumab |
| Track 13 |
Cardiac safety in BCIRG 006 |
| Track 14 |
Magnitude of benefit of anthracyclines in patients with HER2-positive disease |
| Track 15 |
Impact of adjuvant chemotherapy on patients with ER-positive disease |
| Track 16 |
Clinical implications of the adjuvant trastuzumab trial results |
| Track 17 |
Clinical use of delayed adjuvant trastuzumab |
| Track 18 |
Importance of reliable and accurate HER2 testing for treatment decisions |
| Track 19 |
Future adjuvant trials in patients with HER2-positive tumors |
|
|
Select Excerpts from the Interview
 Track 2 Track 2
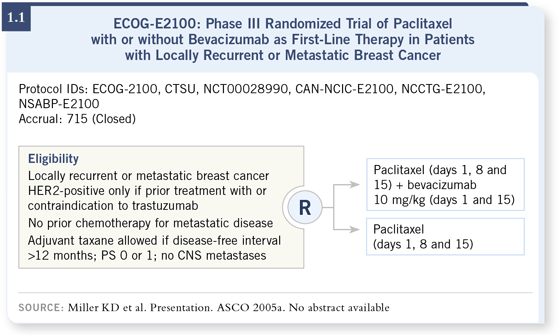
 DR LOVE: Would you comment on the rationale and design of the ECOG-2100 trial? DR LOVE: Would you comment on the rationale and design of the ECOG-2100 trial? |
 DR BURSTEIN: ECOG-2100 was a trial of chemotherapy alone or in combination with bevacizumab as first-line treatment for metastatic breast cancer. Patients were offered weekly paclitaxel three weeks out of four or that same regimen combined with bevacizumab given every two weeks (1.1). DR BURSTEIN: ECOG-2100 was a trial of chemotherapy alone or in combination with bevacizumab as first-line treatment for metastatic breast cancer. Patients were offered weekly paclitaxel three weeks out of four or that same regimen combined with bevacizumab given every two weeks (1.1).
The important thing about this study is that it followed a previous trial that looked at capecitabine with or without bevacizumab for anthracycline- and taxane-treated advanced breast cancer. In that study, which Kathy Miller presented at the San Antonio meeting in 2002 (Miller 2002) and published this spring in the JCO (Miller 2005b), there really had not been a dramatic benefit for adding bevacizumab to chemotherapy.
There has been a temptation to look through the retrospectoscope to say, “That previous study was a positive study because the response rate went from 19 to 30 percent; therefore, we should have known to expect more from bevacizumab.” But those responses were very short lived, and there was no difference in progression-free or overall survival, so I think the question as to whether bevacizumab would be beneficial for advanced breast cancer was unanswered.
 DR LOVE: In that study of capecitabine with or without bevacizumab, patients with HER2-positive disease were eligible. How did that differ from the ECOG-2100 trial? DR LOVE: In that study of capecitabine with or without bevacizumab, patients with HER2-positive disease were eligible. How did that differ from the ECOG-2100 trial?
 DR BURSTEIN: ECOG-2100 ended up being a study principally of HER2- negative breast cancer. Patients with HER2-positive tumors were eligible if for some reason they could not receive trastuzumab, but because most patients can receive trastuzumab, that was a very small component of the study. DR BURSTEIN: ECOG-2100 ended up being a study principally of HER2- negative breast cancer. Patients with HER2-positive tumors were eligible if for some reason they could not receive trastuzumab, but because most patients can receive trastuzumab, that was a very small component of the study.
The other difference is that in ECOG-2100, patients had received less chemotherapy. It was a study for women who had not had any prior chemotherapy for advanced breast cancer. They could have had anthracycline-based chemotherapy or even taxane-based chemotherapy in the adjuvant setting if they were more than one year out from treatment.
 DR LOVE: What were the key findings of the trial? DR LOVE: What were the key findings of the trial?
 DR BURSTEIN: First, it showed that bevacizumab was reasonably well tolerated in the advanced breast cancer population. There is an increased risk of hypertension, which has been seen in some of the other trials. Fortunately, there were relatively few events such as thromboembolism or bleeding complications. A lot of patients had some minor degree of nosebleeds, or epistaxis, but for the most part, the drug proved quite well tolerated. DR BURSTEIN: First, it showed that bevacizumab was reasonably well tolerated in the advanced breast cancer population. There is an increased risk of hypertension, which has been seen in some of the other trials. Fortunately, there were relatively few events such as thromboembolism or bleeding complications. A lot of patients had some minor degree of nosebleeds, or epistaxis, but for the most part, the drug proved quite well tolerated.
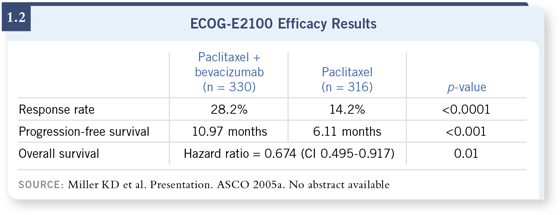
The second important finding was that the study met its primary endpoint, which was to show that adding bevacizumab did improve time to progression (1.2). The time to progression with chemotherapy alone was approximately five to six months, and that nearly doubled to about 11 months with the combination of paclitaxel and bevacizumab.
Associated with that gain in time to progression was an improvement in response rate. The response rate with chemotherapy alone was about 14 percent, and that increased to 28 percent by adding bevacizumab.
There was a suggestion, though it’s very limited follow-up, of an improvement in survival on the order of a couple of months. That difference was statistically significant, though it must be acknowledged that these were preliminary data, and we’ll hear more about that in San Antonio.
 Track 3 Track 3
 DR LOVE: It seems that in metastatic disease trials there has been a trend of focusing on progression-free survival rather than response rate or overall survival. DR LOVE: It seems that in metastatic disease trials there has been a trend of focusing on progression-free survival rather than response rate or overall survival. |
 DR BURSTEIN: Yes, I think that’s true, and the reason for that is complex. First, there is an appreciation that clinical improvement with response is a relatively soft endpoint for most patients. Patients would like to live longer and live free of cancer longer. DR BURSTEIN: Yes, I think that’s true, and the reason for that is complex. First, there is an appreciation that clinical improvement with response is a relatively soft endpoint for most patients. Patients would like to live longer and live free of cancer longer.
Secondly, there has been this theoretical argument that newer drugs that target the vasculature might not actually contribute to response as much as they may simply delay progression. So with some of the drugs that are thought to be inhibitors of tumor differentiation or drugs that might slow down angiogenesis, it has been argued that you might see improvement in progression-free survival without a difference in objective response.
For instance, with bevacizumab in the Phase II trials in renal cell cancer, there were hardly any responses, but there was a dose-dependent difference in time to progression, even though very few patients had objective response (Yang 2003). Interestingly, that has not, as yet, been the case with the more traditional solid tumors in the lung, colon and breast studies. The improvement in progression-free survival has been more or less matched by improvements in response rate.
What’s lacking in all the bevacizumab studies to date is a predictive marker of which patients are likely to benefit and which are not. We don’t have a marker like estrogen receptor or HER2 that would identify patients who are more likely to respond.
In the trial of capecitabine with or without bevacizumab, baseline levels of VEGF expression were evaluated (Miller 2005b). Those levels did not seem to correlate with response or outcome in that initial trial, but, of course, that was a negative trial, so it’s hard to know what to infer.
 Track 4 Track 4
 DR LOVE: What has been your clinical experience with bevacizumab? DR LOVE: What has been your clinical experience with bevacizumab? |
 DR BURSTEIN: We have a lot of clinical trial experience with bevacizumab. We conducted a Phase II study evaluating bevacizumab in combination with vinorelbine. These data were presented at the San Antonio meeting in 2002 and showed that the regimen was reasonably well tolerated and there was clinical activity (Burstein 2002). DR BURSTEIN: We have a lot of clinical trial experience with bevacizumab. We conducted a Phase II study evaluating bevacizumab in combination with vinorelbine. These data were presented at the San Antonio meeting in 2002 and showed that the regimen was reasonably well tolerated and there was clinical activity (Burstein 2002).
There was a response rate of approximately 30 percent. However, at the time, the negative randomized trial with bevacizumab and capecitabine for the refractory patient population was reported, so our trial did not lead to a broad use of our particular combination.
What we’ve been doing more recently is a randomized Phase II study of so-called low-dose or metronomic chemotherapy, given either by itself or in combination with bevacizumab as first-line treatment for advanced breast cancer. We hope to present the data at the 2005 San Antonio meeting (Burstein 2005). We’ve found this regimen very interesting and hope to have some very nice clinical and correlative data to support it.
 DR LOVE: What exactly is the regimen? DR LOVE: What exactly is the regimen?
 DR BURSTEIN: It is 2.5 milligrams of methotrexate twice daily for two days each week plus 50 milligrams of oral cyclophosphamide daily with or without bevacizumab. The argument here is that in laboratory models, the low-dose or metronomic chemotherapy actually provides very good tumor control in many systems and is thought to inhibit angiogenesis factors. DR BURSTEIN: It is 2.5 milligrams of methotrexate twice daily for two days each week plus 50 milligrams of oral cyclophosphamide daily with or without bevacizumab. The argument here is that in laboratory models, the low-dose or metronomic chemotherapy actually provides very good tumor control in many systems and is thought to inhibit angiogenesis factors.
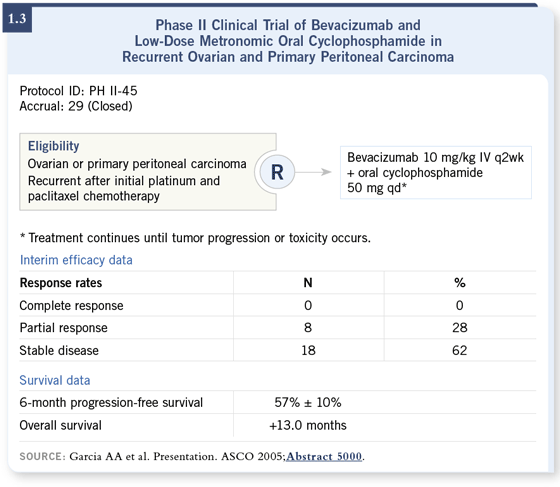
Very exciting data were presented at ASCO 2005 indicating that using lowdose oral cyclophosphamide with bevacizumab led to substantial response in platinum-refractory ovarian cancer (Garcia 2005; [1.3]). This is a very welltolerated combination that I think is worth watching as the data evolve.
 DR LOVE: It appears bevacizumab is relatively patient friendly in the palliative setting, and hopefully we’ll also see that with earlier stage disease. DR LOVE: It appears bevacizumab is relatively patient friendly in the palliative setting, and hopefully we’ll also see that with earlier stage disease.
 DR BURSTEIN: So far that’s been our experience. Bevacizumab doesn’t make you sick to your stomach. It doesn’t make your hair fall out. Patients who’ve been on it for months and months and months do have some hair thinning and some fatigue that accumulates. DR BURSTEIN: So far that’s been our experience. Bevacizumab doesn’t make you sick to your stomach. It doesn’t make your hair fall out. Patients who’ve been on it for months and months and months do have some hair thinning and some fatigue that accumulates.
 DR LOVE: After you complete the Phase II trial of bevacizumab and low-dose oral cyclophosphamide and methotrexate, where do you see this regimen heading? DR LOVE: After you complete the Phase II trial of bevacizumab and low-dose oral cyclophosphamide and methotrexate, where do you see this regimen heading?
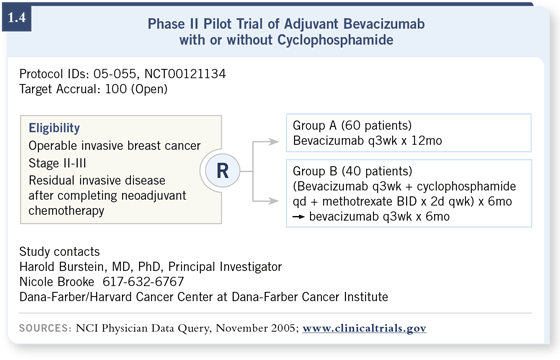
 DR BURSTEIN: The study that we have activated now at Dana-Farber and Indiana University with my good friends Kathy Miller and George Sledge is a pilot study of bevacizumab in the adjuvant setting (1.4). The patient population is women who have had preoperative chemotherapy for breast cancer and who have residual cancer at the time of their surgery. DR BURSTEIN: The study that we have activated now at Dana-Farber and Indiana University with my good friends Kathy Miller and George Sledge is a pilot study of bevacizumab in the adjuvant setting (1.4). The patient population is women who have had preoperative chemotherapy for breast cancer and who have residual cancer at the time of their surgery.
Those women will be offered one year of bevacizumab therapy to see if it’s feasible, and then a second cohort of the same type of patients will be offered one year of bevacizumab and six months of the metronomic chemotherapy.
We chose this patient population for a couple of very specific reasons. First, we know that women who have residual disease after preoperative chemotherapy constitute a very high-risk patient population for whom there is no standard treatment.
Secondly, these women have tumors that, by definition, have some resistance to chemotherapy, so instead of just treating them with more chemotherapy, we thought it would be interesting to bring in a biologic agent.
 DR LOVE: That’s fascinating. Whenever we gather oncologists to discuss difficult cases, patients with residual disease after neoadjuvant chemotherapy are always among the first to be discussed. DR LOVE: That’s fascinating. Whenever we gather oncologists to discuss difficult cases, patients with residual disease after neoadjuvant chemotherapy are always among the first to be discussed.
 DR BURSTEIN: Yes, so there is the temptation to say, “They’ve progressed on anthracyclines and taxanes in the neoadjuvant setting. They have lots of residual disease. What should we give them? Should we give them capecitabine? Should we give them gemcitabine?” DR BURSTEIN: Yes, so there is the temptation to say, “They’ve progressed on anthracyclines and taxanes in the neoadjuvant setting. They have lots of residual disease. What should we give them? Should we give them capecitabine? Should we give them gemcitabine?”
First, there are no data to suggest that more chemotherapy is beneficial in this setting. Secondly, there’s reason to believe that women with tumors like that have disease that is more or less intrinsically resistant to chemotherapy. The temptation to offer more chemotherapy is understandable, but there are a lot of reasons to believe it’s just not going to be very effective.
 DR LOVE: What was the rationale for the bevacizumab-alone arm of that trial? DR LOVE: What was the rationale for the bevacizumab-alone arm of that trial?
 DR BURSTEIN: First, we don’t know that bevacizumab alone would not be effective, and, of course, the adjuvant trials that are going to answer this question ultimately will be large cooperative group studies of chemotherapy with or without bevacizumab. But bevacizumab alone has the advantage of being better tolerated, so when you start talking about extended periods of therapy, it probably is more feasible. DR BURSTEIN: First, we don’t know that bevacizumab alone would not be effective, and, of course, the adjuvant trials that are going to answer this question ultimately will be large cooperative group studies of chemotherapy with or without bevacizumab. But bevacizumab alone has the advantage of being better tolerated, so when you start talking about extended periods of therapy, it probably is more feasible.
Secondly, we just wanted to see if it would be safe to give six to 12 months of bevacizumab in the adjuvant setting.
 DR LOVE: That’s a great trial. I think it’s going to attract a lot of interest. DR LOVE: That’s a great trial. I think it’s going to attract a lot of interest.
 DR BURSTEIN: We also have some very handsome correlative studies built into it, taking advantage of the proteomics research for which Indiana University is very well known, and looking at some other markers of tumor recurrence and endothelial cell biology in which our group is very interested. DR BURSTEIN: We also have some very handsome correlative studies built into it, taking advantage of the proteomics research for which Indiana University is very well known, and looking at some other markers of tumor recurrence and endothelial cell biology in which our group is very interested.
 Track 5 Track 5
 DR LOVE: The key question oncologists have at this point about bevacizumab — assuming the reimbursement issues are resolved — is specifically how it should be utilized in practice. How are you using it clinically? DR LOVE: The key question oncologists have at this point about bevacizumab — assuming the reimbursement issues are resolved — is specifically how it should be utilized in practice. How are you using it clinically? |
 DR BURSTEIN: We now have data for bevacizumab in combination with paclitaxel. We certainly use a lot of weekly paclitaxel as first-line treatment for advanced breast cancer, so for patients who are already receiving paclitaxel, I believe this is clearly the regimen of choice. DR BURSTEIN: We now have data for bevacizumab in combination with paclitaxel. We certainly use a lot of weekly paclitaxel as first-line treatment for advanced breast cancer, so for patients who are already receiving paclitaxel, I believe this is clearly the regimen of choice.
The challenge is how to treat patients in the second- and third-line settings. At present, there really are only minimal data to indicate that bevacizumab is beneficial for such patients.
Another challenge is what to do for those women who received anthracyclines and taxanes in the adjuvant setting. Do you rechallenge them with paclitaxel and bevacizumab? There are two halves to that question. The first is, does bevacizumab actually help these women? We haven’t seen the data as yet broken out as a function of prior taxane therapy.
The second half of the question is should you give the taxane again? Again, we don’t have good answers. If it’s been more than a year, it’s probably reasonable to give the paclitaxel again. Occasionally, we recommend our vinorelbine regimen, because of our Phase II experience with vinorelbine plus bevacizumab (Burstein 2002).
Some people administer capecitabine plus bevacizumab, because, of course, there are safety data for that. On the other hand, those data don’t really suggest that particular combination does all that much compared to capecitabine alone.
These are the types of issues that follow any exciting new drug introduction. We’re all looking forward to more studies, more Phase II trials, to really try and understand how best to utilize this drug for metastatic disease.
 DR LOVE: What about bevacizumab and docetaxel? DR LOVE: What about bevacizumab and docetaxel?
 DR BURSTEIN: There are limited Phase II data on that combination from a preoperative study that Sandy Swain has done and a trial in the treatment of metastatic breast cancer conducted by Charlie Shapiro at Ohio State (Denduluri 2004; Wedam 2004; Ramaswamy 2003). There will be finite response rates and safety data from these Phase II trials. My personal preference, based on the ECOG-2100 data right now, is to use weekly paclitaxel with bevacizumab. DR BURSTEIN: There are limited Phase II data on that combination from a preoperative study that Sandy Swain has done and a trial in the treatment of metastatic breast cancer conducted by Charlie Shapiro at Ohio State (Denduluri 2004; Wedam 2004; Ramaswamy 2003). There will be finite response rates and safety data from these Phase II trials. My personal preference, based on the ECOG-2100 data right now, is to use weekly paclitaxel with bevacizumab.
 Track 6 Track 6
 DR LOVE: What about the combination of bevacizumab and nanoparticle albumin-bound (nab) paclitaxel? DR LOVE: What about the combination of bevacizumab and nanoparticle albumin-bound (nab) paclitaxel? |
 DR BURSTEIN: What’s interesting about nab paclitaxel is that it seems to have some convenience and safety advantages over paclitaxel — it has a lower rate of hypersensitivity reactions, so it eliminates the need for steroid premedication, and the infusion time is shorter. DR BURSTEIN: What’s interesting about nab paclitaxel is that it seems to have some convenience and safety advantages over paclitaxel — it has a lower rate of hypersensitivity reactions, so it eliminates the need for steroid premedication, and the infusion time is shorter.
One of the consequences of the way the drug was registered, though, is that it wasn’t registered in a study that compared milligram-for-milligram equivalence of paclitaxel in the two formulations. The registration study was an every three-week treatment using paclitaxel at 175 mg/m2 versus nab paclitaxel at 260 mg/m2 (1.5).
A consequence of that is that we don’t really have the data to substitute the drug into all the regimens that were discussed at ASCO this year (2005), in particular, weekly paclitaxel when combined with bevacizumab (Miller 2005a) and weekly paclitaxel or every three-week paclitaxel used in the adjuvant trastuzumab trials (Romond 2005a). We need the Phase II data for the safety and feasibility of those combinations and those schedules and we need to find the appropriate nab paclitaxel dose.
Obviously, those trials are being put together. Our group will be conducting a study of dose-dense AC followed by nab paclitaxel, and patients who have HER2-positive tumors will receive the nab paclitaxel with trastuzumab, so we can begin to characterize the safety and feasibility of these regimens. However, for the moment, I think that the data clearly suggest you should just go with paclitaxel.
 DR LOVE: Some of the nab paclitaxel data suggest lower rates of neutropenia. Is that the case, and will you still use growth factors with dose-dense AC DR LOVE: Some of the nab paclitaxel data suggest lower rates of neutropenia. Is that the case, and will you still use growth factors with dose-dense AC 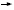 nab paclitaxel? nab paclitaxel?
 DR BURSTEIN: There has been that suggestion of less neutropenia in the literature (Gradishar 2005; [1.5]), and so during our Phase II pilot study, we will be omitting the growth factors with nab paclitaxel for the taxane portion to see if it’s feasible. DR BURSTEIN: There has been that suggestion of less neutropenia in the literature (Gradishar 2005; [1.5]), and so during our Phase II pilot study, we will be omitting the growth factors with nab paclitaxel for the taxane portion to see if it’s feasible.
 Track 10 Track 10
 DR LOVE: Can you summarize the adjuvant trastuzumab studies presented at ASCO and your take on the data? DR LOVE: Can you summarize the adjuvant trastuzumab studies presented at ASCO and your take on the data? |
 DR BURSTEIN: The first presentation was a pooled analysis from the NSABP (NSABP-B-31) and Intergroup trials (NCCTG-N9831; [Romond 2005a]). The second presentation focused on the North American Intergroup study and tackled the question of sequential versus concurrent trastuzumab (Perez 2005). The third presentation was on the European HERA trial, which was a study of no therapy versus one or two years of trastuzumab after adjuvant chemotherapy (Piccart-Gebhart 2005a). DR BURSTEIN: The first presentation was a pooled analysis from the NSABP (NSABP-B-31) and Intergroup trials (NCCTG-N9831; [Romond 2005a]). The second presentation focused on the North American Intergroup study and tackled the question of sequential versus concurrent trastuzumab (Perez 2005). The third presentation was on the European HERA trial, which was a study of no therapy versus one or two years of trastuzumab after adjuvant chemotherapy (Piccart-Gebhart 2005a).
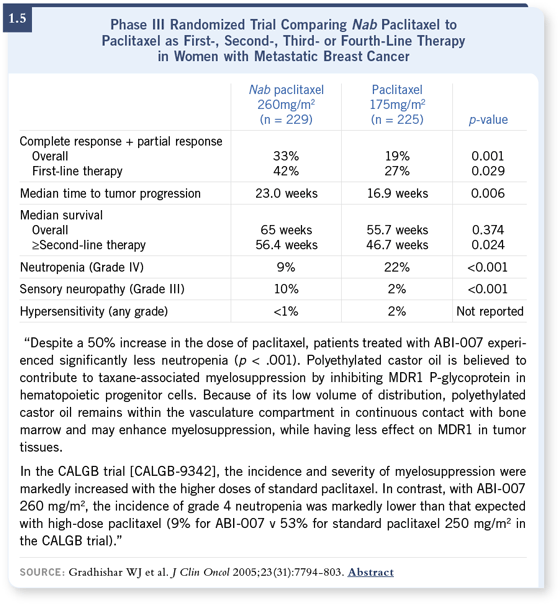
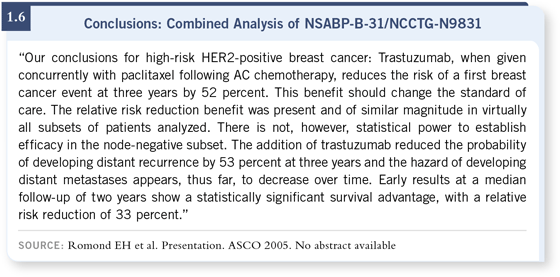
Dr Romond presented the pooled NSABP and Intergroup data. They combined these data because they realized that trial accrual was still ongoing in the adjuvant trastuzumab studies in North America, and there was a sense of urgency to try and get some answers quickly. With the blessing of the National Cancer Institute, the NSABP and the Intergroup decided to pool two very separate trials into one, which would be analyzed for disease-free survival, and asked the question whether AC followed by paclitaxel with or without trastuzumab was better.
The results of the pooled analysis clearly showed that adding trastuzumab lowered the risk of recurrence by about half (1.6, 1.7). There was a very dramatic p-value — something like three times 10 to the negative 12th power — which everyone made a big deal out of, but suffice it to say this was not due to chance. And I think that the relative risk translated to a very substantial clinical gain. The risk of recurrence in the patients who received chemotherapy alone was actually quite high, 25 percent recurrence rate at three years — nearly 33 percent recurrence rate at four years — and, again, adding trastuzumab cut that risk in half.
The patients were randomly assigned to chemotherapy with or without trastuzumab, but half of the women had hormone receptor-positive tumors and presumably had received anti-estrogen therapy as well.
 DR LOVE: Considering the fact that the patients had received adjuvant chemotherapy and, in addition, half of them had received adjuvant hormonal therapy, a 25 percent rate of relapse at three years is pretty impressive. DR LOVE: Considering the fact that the patients had received adjuvant chemotherapy and, in addition, half of them had received adjuvant hormonal therapy, a 25 percent rate of relapse at three years is pretty impressive.
 DR BURSTEIN: It is, and it just underscores how high-risk HER2-positive breast cancers really are. These data, as they were presented, were very clean, and you sit in the back of the amphitheater and say, “Now everything is different,” because you’re going to start offering adjuvant trastuzumab. DR BURSTEIN: It is, and it just underscores how high-risk HER2-positive breast cancers really are. These data, as they were presented, were very clean, and you sit in the back of the amphitheater and say, “Now everything is different,” because you’re going to start offering adjuvant trastuzumab.
 DR LOVE: What are your thoughts about the cardiac toxicity data? DR LOVE: What are your thoughts about the cardiac toxicity data?
 DR BURSTEIN: There are still things we need to learn about this therapy. The price for using trastuzumab is a greater risk of cardiac toxicity, and we need to better understand that risk. That risk was about four percent in the NSABP experience with trastuzumab plus chemotherapy versus less than one percent with chemotherapy alone. It is true that in many instances the cardiac toxicity is reversible, but not always. DR BURSTEIN: There are still things we need to learn about this therapy. The price for using trastuzumab is a greater risk of cardiac toxicity, and we need to better understand that risk. That risk was about four percent in the NSABP experience with trastuzumab plus chemotherapy versus less than one percent with chemotherapy alone. It is true that in many instances the cardiac toxicity is reversible, but not always.
There was a suggestion that women who are older than age 50 and women whose baseline LVEF was borderline, between 50 and 55 percent, might be particularly at risk for cardiac toxicity. Clearly, ongoing cardiac surveillance and careful patient selection is still warranted when using this agent.
There were really two differences between the cardiac screening in the HERA trial and the North American trials. First, in the HERA trial, women were eligible after they’d finished all their chemotherapy, so if they had a major drop in their LVEF during their chemotherapy, they were ineligible for the trial.
Secondly, the threshold in the HERA trial was 55 percent, a little bit higher than in the North American trials. If you actually look in the North American studies at the women whose ejection fractions were greater than 55 percent, the risk of cardiac toxicity looks to be about one to two percent, which is more or less what was seen in the HERA trial.
I don’t know that we have compelling data suggesting that sequential therapy is safer than concurrent therapy with paclitaxel, and I don’t think we know the window for which you need to be finished with your anthracyclines before you start the trastuzumab.
 DR LOVE: The other impressive finding in the combined analysis was that it showed a survival benefit at just two years of follow-up. DR LOVE: The other impressive finding in the combined analysis was that it showed a survival benefit at just two years of follow-up.
 DR BURSTEIN: There was a suggestion of a survival benefit in the pooled analysis. It was about a two to three percent difference at two to three years, and the difference was statistically significant. Neither study alone had a survival advantage, but the advantage of pooling the data was to observe enough events. DR BURSTEIN: There was a suggestion of a survival benefit in the pooled analysis. It was about a two to three percent difference at two to three years, and the difference was statistically significant. Neither study alone had a survival advantage, but the advantage of pooling the data was to observe enough events.
 DR LOVE: I interviewed Ed Romond right after that presentation and asked him about the slide he showed of distant disease-free survival, in which there was a dramatic drop at three or four years of follow-up. What were your thoughts on that? DR LOVE: I interviewed Ed Romond right after that presentation and asked him about the slide he showed of distant disease-free survival, in which there was a dramatic drop at three or four years of follow-up. What were your thoughts on that?
 DR BURSTEIN: I think it is important that most of the events in these trials were distant metastatic events. In the hormone literature, particularly of late, we’ve included all breast cancer events — contralateral tumors, ipsilateral recurrences and distant metastases. That makes sense because hormone receptor-positive tumors have a much more indolent natural history, and these second breast cancer events matter. However, for HER2-positive disease, which is more virulent, really what you’re talking about is preventing distant metastasis, and as you pointed out, even with the short follow-up and even with the availability of trastuzumab in the metastatic setting, there still was a survival difference emerging after just two to three years of follow-up. DR BURSTEIN: I think it is important that most of the events in these trials were distant metastatic events. In the hormone literature, particularly of late, we’ve included all breast cancer events — contralateral tumors, ipsilateral recurrences and distant metastases. That makes sense because hormone receptor-positive tumors have a much more indolent natural history, and these second breast cancer events matter. However, for HER2-positive disease, which is more virulent, really what you’re talking about is preventing distant metastasis, and as you pointed out, even with the short follow-up and even with the availability of trastuzumab in the metastatic setting, there still was a survival difference emerging after just two to three years of follow-up.
 Track 11 Track 11
 DR LOVE: What are your thoughts on the issue of concurrent versus sequential adjuvant chemotherapy and trastuzumab? DR LOVE: What are your thoughts on the issue of concurrent versus sequential adjuvant chemotherapy and trastuzumab? |
 DR BURSTEIN: The context for that really is the global HERA trial, in which women finished all their chemotherapy and radiation therapy and then were randomly assigned to either no further treatment or one or two years of trastuzumab. The data presented were for one year of trastuzumab treatment (Piccart-Gebhart 2005). DR BURSTEIN: The context for that really is the global HERA trial, in which women finished all their chemotherapy and radiation therapy and then were randomly assigned to either no further treatment or one or two years of trastuzumab. The data presented were for one year of trastuzumab treatment (Piccart-Gebhart 2005).
This study also showed a dramatic lowering of the risk of recurrence, again cutting the risk by nearly 50 percent, again with very short follow-up, on average only one to two years, but a statistically and clinically apparent reduction in risk of recurrence. So trastuzumab works at preventing recurrence in the adjuvant setting. Because of the short follow-up, there was no survival advantage as yet reported in the HERA trial.
To circle back to the Intergroup experience, the three arms of the NCCTGN9831 trial were chemotherapy alone, chemotherapy followed in sequence by trastuzumab, or chemotherapy where the patient began trastuzumab concurrent with paclitaxel. In comparing chemotherapy alone versus concurrent chemotherapy/trastuzumab, there was a big difference — a 50 percent risk reduction (Romond 2005a). By contrast, when they compared chemotherapy alone versus chemotherapy followed by sequential trastuzumab, there was a 13 percent risk reduction, which was not statistically significant.
Frankly, it is hard to square the results of the HERA trial with the results of the North American Intergroup study. The most likely explanation is that there is some benefit for sequential trastuzumab therapy, but the benefit is modest. Perhaps the fact that everyone in the Intergroup study received a taxane, compared to the HERA trial in which most women did not receive a taxane, diminished some of the gains that you might see with trastuzumab. Perhaps it’s just an artifact of small numbers of patients with very limited follow-up.
We don’t really know how to optimally use adjuvant trastuzumab. I suspect in North America, most trials moving forward and in clinical practice most physicians will start with concurrent trastuzumab and paclitaxel, as was done in the NSABP and Intergroup studies. Whereas in Europe and around the world, I suspect clinicians will administer trastuzumab after the patient finishes chemotherapy, based on their experience to date.
 Track 16 Track 16
 DR LOVE: The adjuvant trastuzumab data have generated many practical questions as to how to use this agent clinically. How are you using it in your practice? DR LOVE: The adjuvant trastuzumab data have generated many practical questions as to how to use this agent clinically. How are you using it in your practice? |
 DR BURSTEIN: The data were pretty straightforward and, while I think you can quibble with the margins, they’re very simple data — you give patients with HER2-positive tumors trastuzumab. The studies consisted principally of patients with node-positive breast cancer, and so, on the issue of proportional risk reduction and absolute risk reduction, for women at lower risk who have node-negative or hormone receptor-positive breast cancer, we don’t know in absolute terms how much benefit can be gained from adjuvant trastuzumab. DR BURSTEIN: The data were pretty straightforward and, while I think you can quibble with the margins, they’re very simple data — you give patients with HER2-positive tumors trastuzumab. The studies consisted principally of patients with node-positive breast cancer, and so, on the issue of proportional risk reduction and absolute risk reduction, for women at lower risk who have node-negative or hormone receptor-positive breast cancer, we don’t know in absolute terms how much benefit can be gained from adjuvant trastuzumab.
We know from the subset analyses in both the North American trials and the HERA trial that the proportional risk reduction was very similar regardless of nodal status or the number of positive nodes, the size of the tumor, the age of the patient or the hormone receptor status of the tumor (Romond 2005b; Piccart-Gebhart 2005b). All those lined up very similarly in the Forest plots; they all had an average of approximately a 50 percent risk reduction.
Still, that means that you don’t know how much benefit a woman with a 1.2-centimeter, ER-positive, node-negative, HER2-positive tumor receives in absolute terms compared to one who has a three-centimeter, five-positivenode tumor. Those data will be generated, and people will start to weigh in on whether it makes sense or not.
 DR LOVE: In the interim, I expect we will still probably rely on the relative risk reduction concept. DR LOVE: In the interim, I expect we will still probably rely on the relative risk reduction concept.
 DR BURSTEIN: I would think so. The national guideline panels will be rapidly adopting these data. The NCCN has already evaluated the data. I sit on that panel, and they will recommend trastuzumab for all patients with node-positive breast cancer and, for patients with node-negative disease, they will recommend considering trastuzumab for those women who would have met the eligibility criteria for the Intergroup study — a one-centimeter tumor if the tumor were ER-negative or a two-centimeter tumor if it were ER-positive. DR BURSTEIN: I would think so. The national guideline panels will be rapidly adopting these data. The NCCN has already evaluated the data. I sit on that panel, and they will recommend trastuzumab for all patients with node-positive breast cancer and, for patients with node-negative disease, they will recommend considering trastuzumab for those women who would have met the eligibility criteria for the Intergroup study — a one-centimeter tumor if the tumor were ER-negative or a two-centimeter tumor if it were ER-positive.
They say “consider,” as opposed to just do it, only because there are so few data from patients with node-negative disease. Patients with negative nodes comprised roughly five percent of the pooled North American clinical experience, approximately 10 percent of the entire Intergroup trial and about 30 percent of the HERA trial, so there are very little data on node-negative disease.
 Track 17 Track 17
 DR LOVE: One of the most common questions to arise from the adjuvant trastuzumab data is how to treat the patient who is one, two or three years out from diagnosis. What are your thoughts on this? DR LOVE: One of the most common questions to arise from the adjuvant trastuzumab data is how to treat the patient who is one, two or three years out from diagnosis. What are your thoughts on this? |
 DR BURSTEIN: In the North American trials, the risk of recurrence is very pronounced in the first two or three years. If the patient is beyond three years out, her risk is really quite different from what it was at baseline. So aside from the fact that there’s no data on whether to give trastuzumab to women who are two or three years out, it’s not clear that they need the trastuzumab. DR BURSTEIN: In the North American trials, the risk of recurrence is very pronounced in the first two or three years. If the patient is beyond three years out, her risk is really quite different from what it was at baseline. So aside from the fact that there’s no data on whether to give trastuzumab to women who are two or three years out, it’s not clear that they need the trastuzumab.
For women with HER2-positive tumors who’ve just finished chemotherapy within the past several months, we have been suggesting they consider trastuzumab. Obviously, this is a time-limited problem. In patients who are more than six or 12 months out, we have not frequently gone back and added trastuzumab to their treatment regimen. I know that different centers have drawn the line in the sand at different places.
I think that a sobering experience from the Intergroup trial is that there is no statistically significant gain in the Intergroup study in the sequential arm. At best, we are offering a modest advantage to such patients.
Select publications
|

