
 |
||||||||

 “If you’re looking only for big advances, then the only things that will contribute towards your aim are exaggerated claims, over-optimistic claims, claims by people who actually are not being realistic about what they can achieve. Now this is a pity, and it’s particularly a pity in the context of breast cancer because in breast cancer, moderate improvements in prognosis are really worthwhile. They can be humanly worthwhile. They’re not large percentages, but they’re large numbers of human beings.
“If you’re looking only for big advances, then the only things that will contribute towards your aim are exaggerated claims, over-optimistic claims, claims by people who actually are not being realistic about what they can achieve. Now this is a pity, and it’s particularly a pity in the context of breast cancer because in breast cancer, moderate improvements in prognosis are really worthwhile. They can be humanly worthwhile. They’re not large percentages, but they’re large numbers of human beings.
Every year in the United States, there are 400,000 cancer deaths roughly. Of these, about 40,000 involve breast cancer. Now, realistically, the kinds of change that you’re going to hear described today, the kinds of trials that you’re going to hear described, might, if we’re lucky, involve avoidance about say of 4,000 of those 40,000 deaths. Now avoidance of 4,000 deaths is humanly worthwhile. I mean there’s nowhere near 4,000 people in this auditorium, and avoidance of the instant deaths of all of us would certainly be worthwhile.
So, 4,000 deaths is worth knowing about, but it’s a small percentage, and it’s very difficult to pick out those kinds of small percentages. And, so, you’ve really got to have very accurate and very sensitive, large randomized trials.”
Sir Richard Peto
NIH Consensus Conference on Early Breast Cancer
First presentation of the International Trials’ Overview of Breast Cancer
September 9, 1985
“For the first time in recorded history, annual cancer deaths in the United States have fallen.”
Graphic from NIH website noting progress in cancer control as demonstrated
by the decrease in US deaths from 557,271 in 2002 to 556,902 in 2003
When Richard Peto, the boy-genius statistician from Oxford, took the stage in a dusty auditorium in Bethesda more than 20 years ago, he had the Cheshire-like visage of the kid in your class who had the answer no one could figure out — and the cool thing was that it looked so simple.
Prior to this historic first presentation of the International Breast Cancer Overview, a series of smallish clinical trials had evaluated the role of tamoxifen as adjuvant therapy mostly for women with ER-positive, node-positive tumors. Only one or two of these studies demonstrated a statistically significant improvement in survival.
Peto, who worked closely with fellow Brit Richard Doll (both would be knighted by Queen Elizabeth II) fully understood several important aspects of research methodology that very few investigators appreciated at that time, including two key issues related to Phase III randomized clinical trials:
1. The importance of primary study endpoints in clinical trial design
For example, in adjuvant trials, overall survival occupies center stage, but Peto pointed out that it takes longer to see deaths, and there are fewer deaths than recurrences. Today, overall survival surrogates like two- to three-year disease-free survival yield quicker answers, and these in turn lead to the next generation of trials.2. Interpretation of “negative” clinical trials
Peto noted that if a trial does not demonstrate a difference in measured primary endpoints, there are two very different potential explanations: either the therapy does not have an effect on that endpoint, or it does but there are not enough events to be able to detect it.
These observations were profoundly simple and Peto drove his points home in vivid black and white. Like many academicians of that era, he loved transparencies.
Presenting a mind-boggling panorama of graphics utilizing methods that were new to oncologists (eg, Forest plots as in Figure 1), Peto stunned the meeting attendees by demonstrating that when individual trials (and events) were combined, all kinds of interesting effects could be seen.
Peto was also obsessed with the idea of publication bias and as such badgered, pleaded and cajoled every researcher in the world he could identify who had completed a randomized breast cancer trial — positive or negative — to turn over to him the raw data. He and his team then “cleaned up” this information individually on a case-by-case basis to, in effect, create one large clinical trial.
This first meta-analysis instantly validated a major impact of tamoxifen on survival in the adjuvant setting. Within weeks, the NCI released a consensus statement suddenly supporting adjuvant tamoxifen, and as a result, tens of thousands of women received a potentially curative therapy that had previously been considered a “kinder, gentler” way to delay disease progression.
Unfortunately, we needed one more overview to really drive home the events issue. Specifically, when this first tamoxifen overview was broken down by nodal and menopausal status, the benefits observed were statistically significant only in postmenopausal women with positive nodes, prompting the NCI consensus statement to support treatment only in this subset.
In the five years that led up to the next overview in 1990, I interviewed Michael Baum on several occasions, one of Peto’s original overview accomplices (along with Craig Henderson, Bill Wood and others). Mike would routinely become apoplectic when I raised this tamoxifen subset issue, which in retrospect we now see was all about events. Five years later, with more recurrences in both the node-negative and premenopausal subsets, it was clear that tamoxifen worked pretty much the same in all women with ER-positive disease.
So here we are, 22 years later, with a finely honed clinical research machine that produces increasingly gigantic trials, vividly demonstrated by the ATAC and BIG 1-98 trials, which included more than 17,000 patients compared to 16,513 women in 28 trials of tamoxifen in the original 1985 overview.
We now frequently learn at plenary presentations at ASCO and other meetings that new and oftentimes costly novel agents improve progression-free survival in the metastatic setting by a few weeks, and treatment standards quickly adapt as clinical investigators dutifully extol these benefits and CME vehicles like this one get the word out.
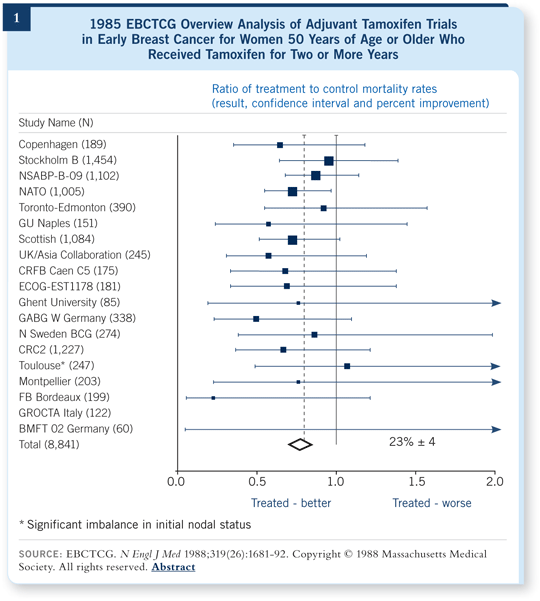
The relative failure of this step-by-step approach to cancer research is evident considering that in 1985, as noted by Sir Richard, there were approximately 400,000 people dying of cancer in the United States every year. Twenty-two years later, the NCI is pleased to report our current annual mortality of more than 550,000 has dropped by one tenth of one percent. With all due respect to the aging of our population, these numbers are going in the wrong direction fast.
I am not here to disparage the meaningful advances we have made, nor our plan for rational molecular targeted therapy. Adjuvant trastuzumab has been a stand-up triple, if not a home run, and I love the imatinib story in CML and GIST as much as anyone. Hopefully, targeted molecular therapy won’t be a dream gone sour like cytotoxic chemotherapy, which is unfortunately a lot less effective in breast cancer and other common tumors than it is for testicular cancer. However, it seems logical that, for this enormous public health problem, there should also be room for a spectrum of interventions, including some with creativity.
Peto’s brilliant observation and his charismatic leadership helped to form the basis of an oncology research strategy where huge trials move the field slowly forward. This approach can and must continue, but at the same time, with apologies to the numbers knight, we also need to be a lot less satisfied with incremental gains and find the resources to investigate new ideas that shoot for the stars.
So what would this new “swing for the fences” approach look like? Well, if I were the “Cancer Czar” empowered with a blank check and a directive to find quicker answers in this endless war, the first step would be to bring together the best minds in the business and start brainstorming. Think of it as cancer’s version of the Manhattan Project, but our objective would be to salvage lives instead of obliterating them.
As a team, we would look at historical examples like ulcer disease, where the Helicobacter model replaced arcane theories such as the “ulcer personality.” We would then start discussing and debating our own ideas. Who knows where this might lead, but off the top of my head I can think of a couple of dozen clinical investigators, including those featured on this issue of Breast Cancer Update, who perhaps could be putting more of their impressive brainpower toward emptying the very clinics they spend so much time running and staffing.
My second major objective would be to develop a “living laboratory” to study this disease that we seem to know so little about. We would launch a massive effort to expand the current tumor registry concept to a level previously unknown.
The goal would be to enlist hundreds of thousands of patients with all tumor types to participate in a huge prospective data-gathering effort. This initiative would include a translational bank of tumor blocks and sera that would be linked to a clinical database comprising electronic medical records and information provided by patients themselves, as discussed on this program by Lee Schwartzberg from The West Clinic in Memphis.
Lee and his extraordinary network of about 500 community-based oncologists gather all types of valuable information from their patients utilizing a simple hand-held, touch-screen computer tablet, and using the same device, they are also able to deliver back educational activities and videos.
My vision would be to employ this innovative technology across the country, in hundreds of oncology offices and cancer centers, to gather data on what patients are eating, how they are exercising, whether they are using supplements and alternative nutraceuticals and to cross-reference these and other data with tumor endpoints and outcomes. Then I would invite my brainy bunch in “Los Alamos” to masticate this outpouring of information and come up with testable hypotheses as to how to intervene in cancer progression.
These are just a few possibilities off the top of my overcrowded head. Undoubtedly there are people way smarter than me who can add to this list if we just let go of the Peto-nian notion that major advances in oncology aren’t possible.
— Neil Love, MD
DrNeilLove@ResearchToPractice.com
October 29, 2007
Select Contributions of Richard Peto and the
Early Breast Cancer Trialists’ Collaborative Group
EDITOR'S NOTE
Peto’s curse
Neil Love, MD
- Select publications
INTERVIEWS
George W Sledge Jr, MD
- Select publications
William J Gradishar, MD
- Select publications
Lee S Schwartzberg, MD
- Select publications
FACULTY TUMOR PANEL
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity