
 |
||||||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 2-3
![]() DR LOVE: Would you describe the novel electronic tool your community research network has been routinely using to collect patient symptom data?
DR LOVE: Would you describe the novel electronic tool your community research network has been routinely using to collect patient symptom data?
![]() DR SCHWARTZBERG: We developed the Patient Care Monitor (PCM), a tablet-based computerized questionnaire with a series of symptom scales that patients complete, via a touch screen, every time they come into the office. It generally takes a patient 10 minutes to finish the survey, whereas it once took a nurse 60 to 90 minutes to glean all of this information.
DR SCHWARTZBERG: We developed the Patient Care Monitor (PCM), a tablet-based computerized questionnaire with a series of symptom scales that patients complete, via a touch screen, every time they come into the office. It generally takes a patient 10 minutes to finish the survey, whereas it once took a nurse 60 to 90 minutes to glean all of this information.
A printout is then handed to the clinician that shows, on a scale of zero to 10, the patient’s new responses, his or her baseline data and the last two determinations. This information shapes the interview because I can instantly identify the real issues. I’ve been using the PCM for approximately seven years, and I find it indispensable. It has been validated and has been tested with the elderly, and we also have a Spanish version.
![]() DR LOVE: How does a physician acquire this system?
DR LOVE: How does a physician acquire this system?
![]() DR SCHWARTZBERG: The current model is available at no cost to the physician.
We supply them through data-reporting projects and currently have 100 practices and 500 doctors around the country using it. Physicians can also correlate PCM data across their practice and we can extract that information to conduct retrospective symptom-assessment studies, which have become powerful because we have this huge database.
DR SCHWARTZBERG: The current model is available at no cost to the physician.
We supply them through data-reporting projects and currently have 100 practices and 500 doctors around the country using it. Physicians can also correlate PCM data across their practice and we can extract that information to conduct retrospective symptom-assessment studies, which have become powerful because we have this huge database.
Track 4
![]() DR LOVE: In a recent Patterns of Care survey we conducted, we learned that 30 percent of physicians in the US who prescribe nab paclitaxel use steroid premedications with it, which surprised us because one of the advantages
of this agent is that this prophylaxis is not required. What patient-reported impact do you see from corticosteroids used as premedications?
DR LOVE: In a recent Patterns of Care survey we conducted, we learned that 30 percent of physicians in the US who prescribe nab paclitaxel use steroid premedications with it, which surprised us because one of the advantages
of this agent is that this prophylaxis is not required. What patient-reported impact do you see from corticosteroids used as premedications?
![]() DR SCHWARTZBERG: We see profound effects from corticosteroids on patient symptoms and behavior, including acute symptoms of insomnia and jitteriness, anxiety, agitation of diabetes and the attendant problems with that. We also see an increase in infections and swelling.
DR SCHWARTZBERG: We see profound effects from corticosteroids on patient symptoms and behavior, including acute symptoms of insomnia and jitteriness, anxiety, agitation of diabetes and the attendant problems with that. We also see an increase in infections and swelling.
![]() DR LOVE: What are your thoughts about the fact that some physicians are still using steroid premedications with this agent?
DR LOVE: What are your thoughts about the fact that some physicians are still using steroid premedications with this agent?
![]() DR SCHWARTZBERG: It’s sobering. I believe it reflects a lack of information getting through and the fact that oncologists today wear many hats and are barraged with information. Through efforts such as yours, it’s become easy for practitioners to receive the information, but the problem is filtering and making sense of all the new data while they are busy taking care of patients.
DR SCHWARTZBERG: It’s sobering. I believe it reflects a lack of information getting through and the fact that oncologists today wear many hats and are barraged with information. Through efforts such as yours, it’s become easy for practitioners to receive the information, but the problem is filtering and making sense of all the new data while they are busy taking care of patients.
We have no good reason to routinely premedicate patients who are treated with nab paclitaxel, and your finding may be due to the inertia of having used corticosteroids with other taxanes.
![]() DR LOVE: In the clinical trials with nab paclitaxel, were the patients premedicated?
DR LOVE: In the clinical trials with nab paclitaxel, were the patients premedicated?
![]() DR SCHWARTZBERG: No, they were not.
DR SCHWARTZBERG: No, they were not.
![]() DR LOVE: Is there a rationale for it with regard to nausea and vomiting?
DR LOVE: Is there a rationale for it with regard to nausea and vomiting?
![]() DR SCHWARTZBERG: I don’t believe so. In my experience, the incidence of nausea and vomiting associated with nab paclitaxel is low, and I believe that according to the NCCN and ASCO guidelines, no reason exists to routinely premedicate these patients. Occasionally we see a patient who has a hypersensitivity reaction or experiences nausea and vomiting, and the lowest level of first-line antiemetic prophylaxis would be steroids alone or a 5-HT3 receptor antagonist alone.
DR SCHWARTZBERG: I don’t believe so. In my experience, the incidence of nausea and vomiting associated with nab paclitaxel is low, and I believe that according to the NCCN and ASCO guidelines, no reason exists to routinely premedicate these patients. Occasionally we see a patient who has a hypersensitivity reaction or experiences nausea and vomiting, and the lowest level of first-line antiemetic prophylaxis would be steroids alone or a 5-HT3 receptor antagonist alone.
Track 10
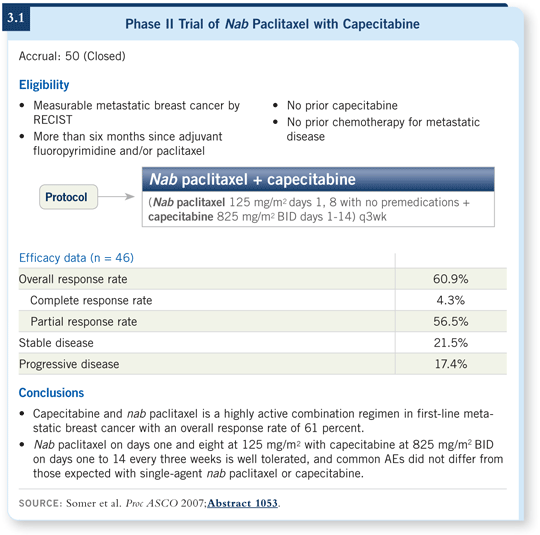
![]() DR LOVE: Can you tell me about your Phase II trial of nab paclitaxel and capecitabine as first-line therapy for metastatic breast cancer?
DR LOVE: Can you tell me about your Phase II trial of nab paclitaxel and capecitabine as first-line therapy for metastatic breast cancer?
![]() DR SCHWARTZBERG: This trial evaluated nab paclitaxel at 125 mg/m2 administered
on days one and eight with capecitabine administered at 825 mg/m2 BID on days one through 14 of a 21-day cycle. We reported on it at San Antonio in 2006 and ASCO in 2007, and now we’re writing it up for publication
(Schwartzberg 2006; Somer 2007).
DR SCHWARTZBERG: This trial evaluated nab paclitaxel at 125 mg/m2 administered
on days one and eight with capecitabine administered at 825 mg/m2 BID on days one through 14 of a 21-day cycle. We reported on it at San Antonio in 2006 and ASCO in 2007, and now we’re writing it up for publication
(Schwartzberg 2006; Somer 2007).
The results are highly favorable for this regimen. We reported a 60.9 percent overall response rate, which was the primary endpoint, and the tolerability was good (3.1). Patients were eligible to continue monotherapy with either drug beyond the initial six months of treatment, and a few patients actually remained on the study up to 12 months after initiation of therapy.
![]() DR LOVE: When Joyce O’Shaughnessy reported on docetaxel/capecitabine, some excitement emerged but people were concerned about the toxicity (O’Shaughnessy 2002). Then Bill Gradishar reported on one study and Joanne Blum on another that evaluated paclitaxel and capecitabine, which seemed less toxic but similar in efficacy (Gradishar 2004; Blum 2006).
DR LOVE: When Joyce O’Shaughnessy reported on docetaxel/capecitabine, some excitement emerged but people were concerned about the toxicity (O’Shaughnessy 2002). Then Bill Gradishar reported on one study and Joanne Blum on another that evaluated paclitaxel and capecitabine, which seemed less toxic but similar in efficacy (Gradishar 2004; Blum 2006).
So a study combining nab paclitaxel and capecitabine seems to make sense. Has this combination been studied before?
![]() DR SCHWARTZBERG: I’m not aware that this particular regimen has been studied previously. A great debate has been waging over the last few years in breast cancer. We have spent a lot of energy — in my opinion undue energy — debating singlet versus doublet therapy.
DR SCHWARTZBERG: I’m not aware that this particular regimen has been studied previously. A great debate has been waging over the last few years in breast cancer. We have spent a lot of energy — in my opinion undue energy — debating singlet versus doublet therapy.
In my opinion, it doesn’t matter whether therapy is a singlet or a doublet — what matters is the toxicity. If a comparison of drug A versus drug B showed B had the same toxicity as A yet doubled the progression-free survival, we’d pick B every time. If B happened to be two agents, what difference would it make?
I’m still a believer that doublet therapy is reasonable for a subset of patients, such as those who have visceral disease that is rapidly progressing, in whom we want the best response as soon as possible. That’s not every patient with metastatic breast cancer, but patients come into my clinic every day who I believe can benefit from the most effective therapy as defined by response rate.
Track 11
![]() DR LOVE: What is your algorithm for treating patients with triple-negative metastatic disease?
DR LOVE: What is your algorithm for treating patients with triple-negative metastatic disease?
![]() DR SCHWARTZBERG: We are currently conducting a large Phase II trial that is evaluating docetaxel and bevacizumab (with trastuzumab if HER2-positive), so that study would be my first-line approach for a patient with triple-negative breast cancer.
DR SCHWARTZBERG: We are currently conducting a large Phase II trial that is evaluating docetaxel and bevacizumab (with trastuzumab if HER2-positive), so that study would be my first-line approach for a patient with triple-negative breast cancer.
Outside of a clinical trial, if a patient has triple-negative breast cancer with visceral disease and/or is symptomatic, I generally use a taxane and bevacizumab, based on the ECOG-E2100 data (Miller 2005).
To some extent it depends on what the patient received as adjuvant therapy. In my practice the adjuvant treatment of choice has been dose-dense AC/paclitaxel, so if a patient relapses within 24 months, off protocol I use docetaxel and bevacizumab.
![]() DR LOVE: Have you considered using a combination of either paclitaxel or nab paclitaxel with capecitabine and bevacizumab?
DR LOVE: Have you considered using a combination of either paclitaxel or nab paclitaxel with capecitabine and bevacizumab?
![]() DR SCHWARTZBERG: For our next Phase II trial, we are considering using our nab paclitaxel and capecitabine regimen, which was well tolerated, and adding bevacizumab.
DR SCHWARTZBERG: For our next Phase II trial, we are considering using our nab paclitaxel and capecitabine regimen, which was well tolerated, and adding bevacizumab.
Tracks 13, 16
![]() DR LOVE: What is the rationale behind your new trial combining fulvestrant and capecitabine for patients with hormone receptor-positive advanced breast cancer?
DR LOVE: What is the rationale behind your new trial combining fulvestrant and capecitabine for patients with hormone receptor-positive advanced breast cancer?
![]() DR SCHWARTZBERG: We’re just launching that study now, but I considered it approximately 18 months ago when I reviewed the literature and found that no work had been done on combining chemotherapy and hormonal therapy on a clinical level for at least 20 years. The work that was conducted previously suggesting some signals of antagonism was with tamoxifen — a less effective hormonal therapy if you will — and often CMF or CMF-like chemotherapy. In addition, study designs used 20 to 25 years ago are different from what we would use today. I believe that it’s worth exploring again.
DR SCHWARTZBERG: We’re just launching that study now, but I considered it approximately 18 months ago when I reviewed the literature and found that no work had been done on combining chemotherapy and hormonal therapy on a clinical level for at least 20 years. The work that was conducted previously suggesting some signals of antagonism was with tamoxifen — a less effective hormonal therapy if you will — and often CMF or CMF-like chemotherapy. In addition, study designs used 20 to 25 years ago are different from what we would use today. I believe that it’s worth exploring again.
Today most patients with hormone receptor-positive disease receive an aromatase inhibitor in the adjuvant setting. However, we have few data on how well patients fare whose disease recurs or progresses after they receive an adjuvant aromatase inhibitor. If you extrapolate and examine some of the data, including Bill Gradishar’s EFECT study (Gradishar 2006; [2.3, page 25]), you see that the time to progression for these patients is short. In fact, 50 percent of them have failed by four months.
Our paradigm for decades has been to begin a patient on hormonal therapy to buy as much time as possible before starting chemotherapy. However, if half of the patients are experiencing only four months of progression-free survival, we’re not providing benefit to the majority.
Fulvestrant by itself may be more effective, but we don’t anticipate that because the patients in our study will be somewhat hormone resistant to an aromatase inhibitor. The downregulation of the receptor alone may help to some degree, particularly in certain groups such as patients with HER2-positive breast cancer, but we don’t know that.
The question is, is it beneficial to administer chemotherapy and hormonal therapy? It harkens back to the argument about whether to use a doublet versus a singlet. I believe it comes down to a toxicity issue. If you can administer an oral drug that is well tolerated, doesn’t bring a lot of toxicity and prolongs the time until the patient receives IV chemotherapy, then you might see some benefit.
![]() DR LOVE: How is the capecitabine administered in this trial?
DR LOVE: How is the capecitabine administered in this trial?
![]() DR SCHWARTZBERG: The capecitabine dosing is novel because it’s metronomic
in the sense that it’s a fixed, relatively low dose of 1,500 milligrams per day administered continuously with the fulvestrant. Even though this study was designed before we saw the XCaliBr data, I was encouraged by the fact that capecitabine, at least in combination with bevacizumab, seemed to benefit patients with estrogen receptor-positive breast cancer much more than receptor-negative disease (Sledge 2007).
DR SCHWARTZBERG: The capecitabine dosing is novel because it’s metronomic
in the sense that it’s a fixed, relatively low dose of 1,500 milligrams per day administered continuously with the fulvestrant. Even though this study was designed before we saw the XCaliBr data, I was encouraged by the fact that capecitabine, at least in combination with bevacizumab, seemed to benefit patients with estrogen receptor-positive breast cancer much more than receptor-negative disease (Sledge 2007).
![]() DR LOVE: In this protocol, do you use a loading dose of fulvestrant?
DR LOVE: In this protocol, do you use a loading dose of fulvestrant?
![]() DR SCHWARTZBERG: Yes. The loading-dose strategy we use is to administer 500 milligrams on day one, 250 milligrams on days 14 and 28 and then 250 milligrams monthly. That’s based on pharmacokinetic data that show that the label dose, 250 milligrams every 28 days, takes several months to reach a steady state (Robertson 2007). In that case, we may not achieve target concentrations
of the drug for three or four months and many patients’ disease may have progressed by that time. This loading-dose strategy has become the standard in my practice.
DR SCHWARTZBERG: Yes. The loading-dose strategy we use is to administer 500 milligrams on day one, 250 milligrams on days 14 and 28 and then 250 milligrams monthly. That’s based on pharmacokinetic data that show that the label dose, 250 milligrams every 28 days, takes several months to reach a steady state (Robertson 2007). In that case, we may not achieve target concentrations
of the drug for three or four months and many patients’ disease may have progressed by that time. This loading-dose strategy has become the standard in my practice.
![]() DR LOVE: Have you encountered any problems in terms of reimbursement for the loading-dose strategy?
DR LOVE: Have you encountered any problems in terms of reimbursement for the loading-dose strategy?
![]() DR SCHWARTZBERG: No, we haven’t had any problems with that.
DR SCHWARTZBERG: No, we haven’t had any problems with that.
EDITOR'S NOTE
Peto’s curse
Neil Love, MD
- Select publications
INTERVIEWS
George W Sledge Jr, MD
- Select publications
William J Gradishar, MD
- Select publications
Lee S Schwartzberg, MD
- Select publications
FACULTY TUMOR PANEL
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity