
 |
||||||||

| Tracks 1-27 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 3, 7
![]() DR LOVE: What’s your take on the issue of the optimal chemotherapy regimen to combine with trastuzumab?
DR LOVE: What’s your take on the issue of the optimal chemotherapy regimen to combine with trastuzumab?
![]() DR SLEDGE: With the recent three-year update from the BCIRG 006 trial, we have evidence that TCH appears to be roughly similar to AC
DR SLEDGE: With the recent three-year update from the BCIRG 006 trial, we have evidence that TCH appears to be roughly similar to AC![]() TH in terms of clinical outcome for risk of recurrence, although we still have relatively early follow-up (Slamon 2006).
TH in terms of clinical outcome for risk of recurrence, although we still have relatively early follow-up (Slamon 2006).
It’s possible that the curves might diverge somewhat, but it’s reassuring to have a nonanthracycline-containing approach to treating patients. The major controversy raging right now is whether we should be using anthracyclines for anyone who has HER2-positive early breast cancer.
![]() DR LOVE: What are you doing right now in your own practice outside of a protocol setting?
DR LOVE: What are you doing right now in your own practice outside of a protocol setting?
![]() DR SLEDGE: A year ago, based on the data we had at that time with what appeared to be a somewhat premature analysis of BCIRG 006, I routinely recommended AC
DR SLEDGE: A year ago, based on the data we had at that time with what appeared to be a somewhat premature analysis of BCIRG 006, I routinely recommended AC![]() TH to patients because I thought the curves did not yet support the idea that TCH was equivalent (Slamon 2005).
TH to patients because I thought the curves did not yet support the idea that TCH was equivalent (Slamon 2005).
With maturation of the data and what looks like at least approximate equivalence (Slamon 2006), I now routinely talk to patients about TCH as an alternative.
My experience with disease-free survival curves is that once you’re out two or three years — certainly for a disease like HER2-positive early-stage breast cancer — you have a fair number of events and those curves begin to appear mature.
![]() DR LOVE: What’s your take on the cardiac safety of TCH?
DR LOVE: What’s your take on the cardiac safety of TCH?
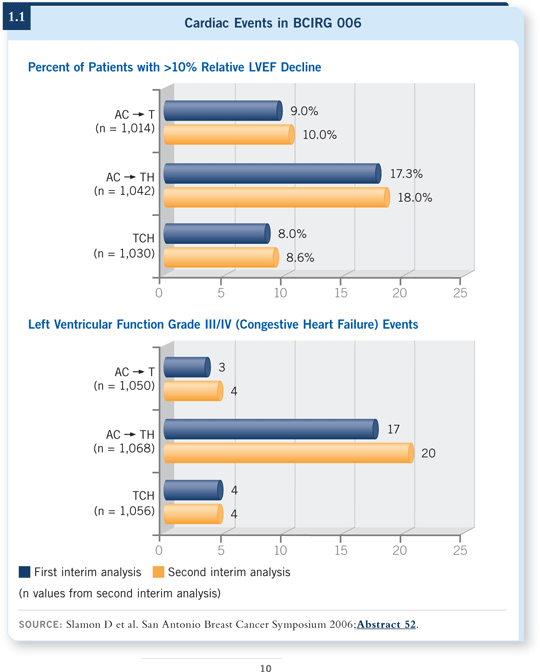
![]() DR SLEDGE: Occasional cases of congestive heart failure occurred in the population of patients who received TCH. Having said that, it’s a low risk — maybe 0.5 percent (1.1).
DR SLEDGE: Occasional cases of congestive heart failure occurred in the population of patients who received TCH. Having said that, it’s a low risk — maybe 0.5 percent (1.1).
Tracks 6-7
![]() DR LOVE: What’s the natural history of smaller, node-negative, HER2-positive tumors, and do you treat those patients with trastuzumab?
DR LOVE: What’s the natural history of smaller, node-negative, HER2-positive tumors, and do you treat those patients with trastuzumab?
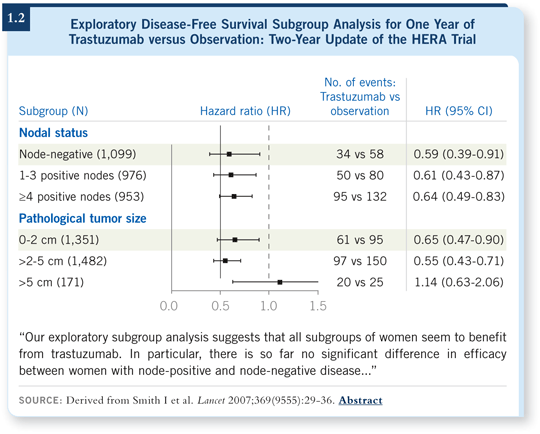
![]() DR SLEDGE: This is what I call the “how low do you go” issue. If we evaluate the clinical trials that we have available in the adjuvant setting, we see that the HERA trial routinely allowed patients with lymph node-negative disease, which represents approximately a third of their population (Piccart-Gebhart 2005; [1.2]).
DR SLEDGE: This is what I call the “how low do you go” issue. If we evaluate the clinical trials that we have available in the adjuvant setting, we see that the HERA trial routinely allowed patients with lymph node-negative disease, which represents approximately a third of their population (Piccart-Gebhart 2005; [1.2]).
The NSABP trial did not allow patients with lymph node-negative disease, and the North Central trial only allowed those patients toward the end of the study recruitment. Approximately 10 to 12 percent of the North Central population and five to six percent of the joint analysis (NSABP-B-31 and NCCTG 9831) had lymph node-negative breast cancer (Romond 2005).
That’s too few patients for a valid subset analysis, but it’s reasonable to ask whether biology would be less important than nodal status in these patients. My bias is that HER2-positive, node-negative breast cancer can kill you and can metastasize just as well as HER2-positive, node-positive cancer.
The issue is further complicated when these tumors are in the subcentimeter size range, for which we have vanishing few data in the clinic. To be honest, many of our “guesstimates” about risk of recurrence are just that.
The databases we have for small, node-negative, HER2-positive tumors are limited, in part because HER2-positive tumors are more likely to be larger. Therefore, it will be difficult to generate a data set that might allow us to examine this issue.
![]() DR LOVE: What would you say to a patient who has a 3- to 5-mm node-negative tumor who might be considering TCH in terms of the risk of clinically significant heart problems that wouldn't otherwise be a factor?
DR LOVE: What would you say to a patient who has a 3- to 5-mm node-negative tumor who might be considering TCH in terms of the risk of clinically significant heart problems that wouldn't otherwise be a factor?
![]() DR SLEDGE: In the ballpark of one half of a percent. This question has actually come up in my clinic for patients with subcentimeter tumors. For instance, I remember distinctly sharing data with a relatively young woman who had subcentimeter, HER2-positive, lymph node-negative breast cancer.
DR SLEDGE: In the ballpark of one half of a percent. This question has actually come up in my clinic for patients with subcentimeter tumors. For instance, I remember distinctly sharing data with a relatively young woman who had subcentimeter, HER2-positive, lymph node-negative breast cancer.
The most compelling issue for her was that her father died of congestive heart failure as a “cardiac cripple” in bed for several years. So this will be the type of negotiation between patient and physician that we’ve dealt with in other adjuvant settings.
Track 8
![]() DR LOVE: Do you think physicians are less confident now in the activity of hormonal therapy when the patient has hormone receptor-positive, HER2-positive disease?
DR LOVE: Do you think physicians are less confident now in the activity of hormonal therapy when the patient has hormone receptor-positive, HER2-positive disease?
![]() DR SLEDGE: If you believe the disease-free survival data in the adjuvant trastuzumab trials, then the primary driver of the biology of these breast cancer types is HER2.
DR SLEDGE: If you believe the disease-free survival data in the adjuvant trastuzumab trials, then the primary driver of the biology of these breast cancer types is HER2.
More important, the other data that have influenced me are from the TAnDEM trial for patients with ER-positive, HER2-positive, metastatic breast cancer receiving front-line hormonal therapy with an aromatase inhibitor, our current best hormonal therapy (Mackey 2006). The median progression- free survival was 2.4 months for the overall population, so hormonal therapy alone does not work well in these patients.
![]() DR LOVE: Does that push the bar lower in terms of using adjuvant trastuzumab?
DR LOVE: Does that push the bar lower in terms of using adjuvant trastuzumab?
![]() DR SLEDGE: Yes, that tends to reinforce the importance of HER2 and anti-HER2 therapy, even in ER-positive tumors.
DR SLEDGE: Yes, that tends to reinforce the importance of HER2 and anti-HER2 therapy, even in ER-positive tumors.
Tracks 9-10
![]() DR LOVE: Can you discuss the ALTTO adjuvant trial for patients with HER2-positive early breast cancer?
DR LOVE: Can you discuss the ALTTO adjuvant trial for patients with HER2-positive early breast cancer?
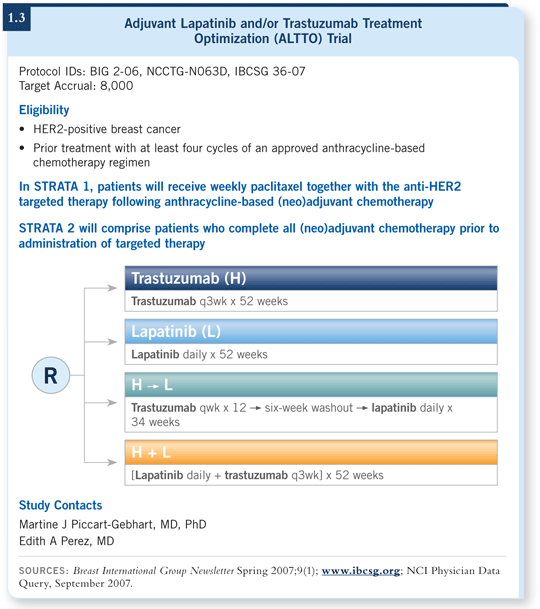
![]() DR SLEDGE: ALTTO is an 8,000-patient, four-arm trial in which patients receive chemotherapy followed by paclitaxel with trastuzumab, lapatinib, the combination or the sequence (1.3). This is a large enough undertaking that it was thought to require two continents’ worth of breast cancer patients to complete.
DR SLEDGE: ALTTO is an 8,000-patient, four-arm trial in which patients receive chemotherapy followed by paclitaxel with trastuzumab, lapatinib, the combination or the sequence (1.3). This is a large enough undertaking that it was thought to require two continents’ worth of breast cancer patients to complete.
![]() DR LOVE: One of the key controversies about this study is that some patients will not receive trastuzumab. Will physicians enroll patients with lower-risk disease but be nervous about patients with high-risk breast cancer?
DR LOVE: One of the key controversies about this study is that some patients will not receive trastuzumab. Will physicians enroll patients with lower-risk disease but be nervous about patients with high-risk breast cancer?
![]() DR SLEDGE: I’m completely comfortable with a nontrastuzumab arm, but many of my colleagues are not. The initial study with lapatinib was for patients whose disease had progressed on trastuzumab, and in that setting lapatinib was clearly beneficial (Geyer 2006).
DR SLEDGE: I’m completely comfortable with a nontrastuzumab arm, but many of my colleagues are not. The initial study with lapatinib was for patients whose disease had progressed on trastuzumab, and in that setting lapatinib was clearly beneficial (Geyer 2006).
A second issue, albeit with smaller trials, is that lapatinib monotherapy produces response rates in the metastatic setting that are equivalent to trastuzumab monotherapy (Vogel 2002).
Third, we now have data that were presented at the last ASCO meeting that reflect one of those fascinating natural biologic experiments. This was a population of patients who received paclitaxel or paclitaxel with lapatinib and whose disease was said to be either HER2-negative or HER2-unknown when they entered the trial (Di Leo 2007).
Based on where the trial was conducted, HER2 testing was not routine, and it turned out that a substantial proportion of patients entering this trial in fact had HER2-positive tumors when central testing was performed.
So an experiment was conducted inside this larger “HER2-negative trial” that allowed us to see what happened with paclitaxel and lapatinib versus paclitaxel for patients with HER2-positive disease, and the results are strikingly positive for the combination in terms of progression-free survival. The results are similar to those of the larger, pivotal trastuzumab trial (Slamon 2001).
Track 13
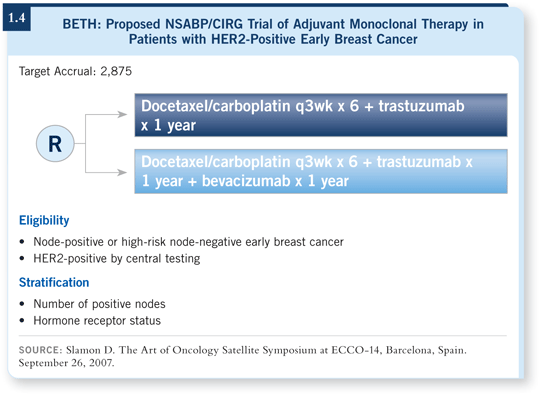
![]() DR LOVE: Another strategy in HER2-positive disease that might have promise is adding bevacizumab to chemotherapy/trastuzumab, an investigational
approach that the NSABP and BCIRG have been discussing (1.4). What are your thoughts about that study?
DR LOVE: Another strategy in HER2-positive disease that might have promise is adding bevacizumab to chemotherapy/trastuzumab, an investigational
approach that the NSABP and BCIRG have been discussing (1.4). What are your thoughts about that study?
![]() DR SLEDGE: That trial has been designed to evaluate a chemotherapy backbone with trastuzumab, and my understanding at present is that it will be TCH with or without bevacizumab.
DR SLEDGE: That trial has been designed to evaluate a chemotherapy backbone with trastuzumab, and my understanding at present is that it will be TCH with or without bevacizumab.
![]() DR LOVE: What do we know about angiogenesis and HER2-positive tumors?
DR LOVE: What do we know about angiogenesis and HER2-positive tumors?
![]() DR SLEDGE: We know that HER2 is an upstream regulator of VEGF production. That has been shown definitively both in cell lines and in the clinic. A woman who has HER2-positive breast cancer simply has more VEGF in her tumor.
DR SLEDGE: We know that HER2 is an upstream regulator of VEGF production. That has been shown definitively both in cell lines and in the clinic. A woman who has HER2-positive breast cancer simply has more VEGF in her tumor.
In some lovely preclinical modeling that was presented in Nature a few years ago, the HER2-positive tumors were considerably more vascular than the HER2-negative tumors (Izumi 2002).
From a clinical standpoint, these tumors have a higher microvessel density. More importantly, VEGF expression is a clear regulator of outcome. In a study conducted by Gottfried Konecny at UCLA analyzing a German database, the tumors with the worst performance were the ones that were both HER2-positive and VEGF-positive (Konecny 2004), so that combination of HER2 positivity and VEGF overexpression appears to be clinically important.
Track 17
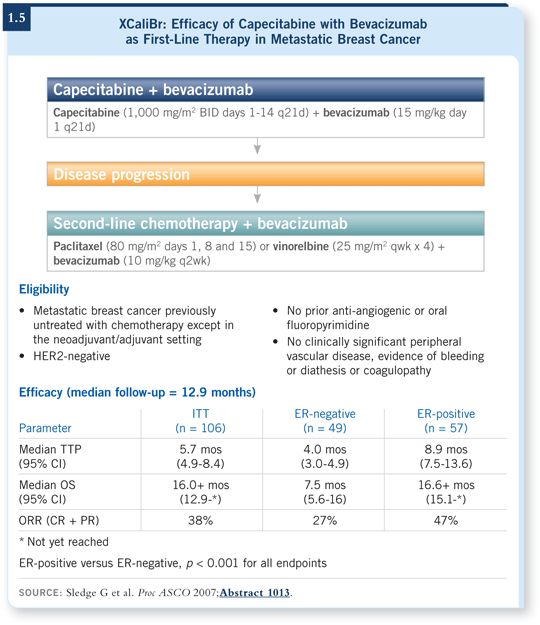
![]() DR LOVE: Could you review the data you presented at ASCO with capecitabine and bevacizumab as first-line therapy in metastatic disease (Sledge 2007)?
DR LOVE: Could you review the data you presented at ASCO with capecitabine and bevacizumab as first-line therapy in metastatic disease (Sledge 2007)?
![]() DR SLEDGE: Several years ago, Kathy Miller presented the ECOG-E2119 trial, which randomly assigned patients with anthracycline- and taxane-refractory
metastatic breast cancer to receive either capecitabine or capecitabine with bevacizumab (Miller 2005a). That trial showed a statistically significant improvement in response rate but no improvement in the primary endpoint of progression-free survival. So Kathy appropriately called that a negative trial when she presented it.
DR SLEDGE: Several years ago, Kathy Miller presented the ECOG-E2119 trial, which randomly assigned patients with anthracycline- and taxane-refractory
metastatic breast cancer to receive either capecitabine or capecitabine with bevacizumab (Miller 2005a). That trial showed a statistically significant improvement in response rate but no improvement in the primary endpoint of progression-free survival. So Kathy appropriately called that a negative trial when she presented it.
The question that came up for me was, is this a case of “nice drugs getting beaten up in bad neighborhoods”? Or was it possible that capecitabine/bevacizumab was simply not a good combination for whatever reason, or perhaps more appropriately, is a taxane with bevacizumab a better or more synergistic combination?
To examine this issue, we launched a multicenter study, XCaliBr, with patients who in essence were similar to the patients who went into ECOG-E2100, which evaluated paclitaxel and bevacizumab (Miller 2005b). They had HER2-negative breast cancer and were receiving their first chemotherapy for metastatic disease.
These patients were treated in a Phase II, single-arm setting, and all received the combination of bevacizumab and capecitabine until progression. At the time of progression, they crossed over and continued to receive bevacizumab with either a taxane — paclitaxel — or vinorelbine.
We have data from the first portion of that trial, and the median progression-free survival for patients receiving bevacizumab and capecitabine was 5.7 months (1.5), which is a disappointing result compared to the 11 months that we saw with the combination of paclitaxel/bevacizumab in E2100.
![]() DR LOVE: Did these patients have worse disease than those in E2100?
DR LOVE: Did these patients have worse disease than those in E2100?
![]() DR SLEDGE: They were slightly different, with a higher proportion that had received prior adjuvant therapy and a higher rate of estrogen receptor (ER) negativity than those who entered E2100.
DR SLEDGE: They were slightly different, with a higher proportion that had received prior adjuvant therapy and a higher rate of estrogen receptor (ER) negativity than those who entered E2100.
One always has to be excruciatingly careful about making too much of an unplanned retrospective subset analysis on small, Phase II trials, but a fascinating observation was that the population with ER-negative disease did extremely poorly. They had a progression-free survival of less than four months and a median overall survival of 7.5 months.
However, the patients with ER-positive disease fared considerably better, with a median progression-free survival in excess of eight months and overall survival in excess of 16 months (1.5), although the median survival has not yet been reached.
Track 22
![]() DR LOVE: Can you discuss the upcoming ECOG trial that will be evaluating
bevacizumab in the adjuvant setting for HER2-negative disease?
DR LOVE: Can you discuss the upcoming ECOG trial that will be evaluating
bevacizumab in the adjuvant setting for HER2-negative disease?
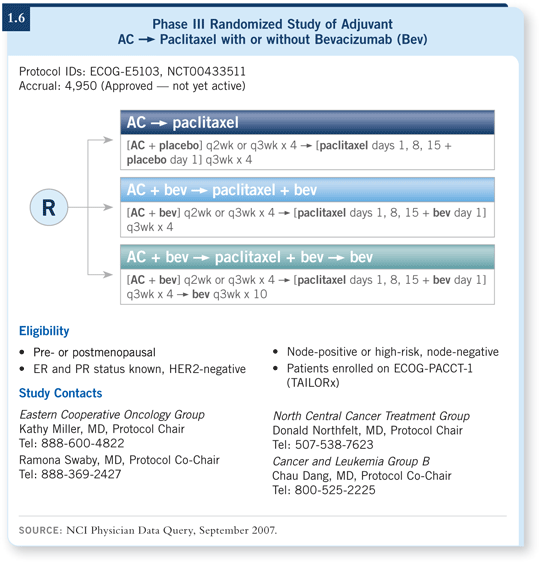
![]() DR SLEDGE: ECOG-E5103 will be a trial within the Intergroup and will accrue approximately 5,000 patients with node-positive or high-risk node-negative, HER2-negative disease (1.6). We have incorporated the Oncotype DX assay into the definition of low risk and high risk for patients with hormone receptor-positive, node-negative disease.
DR SLEDGE: ECOG-E5103 will be a trial within the Intergroup and will accrue approximately 5,000 patients with node-positive or high-risk node-negative, HER2-negative disease (1.6). We have incorporated the Oncotype DX assay into the definition of low risk and high risk for patients with hormone receptor-positive, node-negative disease.
This is a three-arm trial that uses AC followed by weekly paclitaxel as a backbone. The second arm uses the same chemotherapy but adds bevacizumab only during the course of the chemotherapy, beginning with the anthracycline. The third arm uses bevacizumab during chemotherapy and out to a total of one year.
So first we are asking the proof-of-concept question, does bevacizumab add benefit above and beyond adjuvant chemotherapy? And for the second question, is duration of bevacizumab important?
One possibility is that if bevacizumab works, most of its benefit may come as a modifier or a chemopotentiator against endothelial cells. The second biologic possibility is that continued suppression of VEGF is required to prevent microscopic metastases developing into gross metastases.
It’s worth pointing out that we have already conducted an adjuvant pilot trial, ECOG-E2104, which evaluated the combination of bevacizumab with AC followed by paclitaxel, with a particular interest in cardiotoxicity.
Patients were subject to fairly significant cardiac monitoring to explore whether any cardiac signal is emitted by bevacizumab in combination with the anthracycline.
We are still analyzing those data, and we have recorded some cases of congestive heart failure in patients receiving AC with bevacizumab. We have not yet seen enough events to cross our boundary for concern in terms of using the combination in ECOG-E5103, but we are following the data carefully.
EDITOR'S NOTE
Peto’s curse
Neil Love, MD
- Select publications
INTERVIEWS
George W Sledge Jr, MD
- Select publications
William J Gradishar, MD
- Select publications
Lee S Schwartzberg, MD
- Select publications
FACULTY TUMOR PANEL
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity