
 |
||||||||

| Tracks 1-18 | ||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1, 4-5

![]() DR LOVE: Joyce, how would you approach patients with significant residual disease at surgery after neoadjuvant chemotherapy?
DR LOVE: Joyce, how would you approach patients with significant residual disease at surgery after neoadjuvant chemotherapy?
![]() DR O’SHAUGHNESSY: For a patient like this, with triple-negative breast cancer and a fair amount of tumor burden, I order a PET scan up front. It is particularly useful in highly proliferative tumors with a lot of axillary adenopathy,
where we sometimes observe lymphadenopathy in the intramammary chain, in the low cervical nodes and even in the mediastinum. If the disease is chemotherapy sensitive and you have a chance for a decent prognosis, then you can include those nodal beds in the radiation port.
DR O’SHAUGHNESSY: For a patient like this, with triple-negative breast cancer and a fair amount of tumor burden, I order a PET scan up front. It is particularly useful in highly proliferative tumors with a lot of axillary adenopathy,
where we sometimes observe lymphadenopathy in the intramammary chain, in the low cervical nodes and even in the mediastinum. If the disease is chemotherapy sensitive and you have a chance for a decent prognosis, then you can include those nodal beds in the radiation port.
Second, if we can document metastatic disease, we may have the opportunity to use bevacizumab outside of a clinical trial if the patient does not show a complete pathologic response to neoadjuvant therapy.
![]() DR BURSTEIN: This patient received a baseline PET scan that suggested disease in the breast and ipsilateral axilla in addition to equivocal findings in some of the other regional lymph nodes, including the contralateral axilla. In this case, the PET scan muddied matters more than clarifying them, and she had been treated with curative intent.
DR BURSTEIN: This patient received a baseline PET scan that suggested disease in the breast and ipsilateral axilla in addition to equivocal findings in some of the other regional lymph nodes, including the contralateral axilla. In this case, the PET scan muddied matters more than clarifying them, and she had been treated with curative intent.
![]() DR LOVE: How do you usually treat patients like this outside of a clinical trial?
DR LOVE: How do you usually treat patients like this outside of a clinical trial?
![]() DR O’SHAUGHNESSY: I generally use noncross-resistant chemotherapy agents in cases like this. In the absence of any other data, I turn to agents with either a proven track record in the adjuvant setting or for which there is evidence of some preoperative activity.
DR O’SHAUGHNESSY: I generally use noncross-resistant chemotherapy agents in cases like this. In the absence of any other data, I turn to agents with either a proven track record in the adjuvant setting or for which there is evidence of some preoperative activity.
I would probably choose capecitabine combined with a platinum agent and bevacizumab. Capecitabine is a noncross-resistant drug, and work from Farber and others suggests it has some activity in triple-negative disease. I would try to give the patient the best chance for cytoreduction and disruption of any micrometastatic niche she might have.
![]() DR LOVE: Hal, did this patient receive any further treatment after surgery?
DR LOVE: Hal, did this patient receive any further treatment after surgery?
![]() DR BURSTEIN: She received radiation therapy to the breast and regional lymph nodes after her chemotherapy, but she did not receive further systemic therapy after surgery. She was followed expectantly and within three or four months developed changes in the ipsilateral breast. She had a red rash, which was thought to be a cellulitis, and she received a course of antibiotics.
DR BURSTEIN: She received radiation therapy to the breast and regional lymph nodes after her chemotherapy, but she did not receive further systemic therapy after surgery. She was followed expectantly and within three or four months developed changes in the ipsilateral breast. She had a red rash, which was thought to be a cellulitis, and she received a course of antibiotics.
A skin biopsy confirmed recurrence and a repeat staging PET and CT scan showed changes in the left breast, the left supraclavicular lymph node chain, the right axilla, some mediastinal nodes, a “hot” pericardiac node and perhaps a bone lesion.
She received two cycles of docetaxel at 75 milligrams and carboplatin at an AUC of six. She developed febrile neutropenia with the first cycle, so with the second cycle she received growth factor support. She had a partial clinical response in that the erythema decreased and some of the lymph node burden improved clinically. She then saw me as a second opinion in the case.
![]() DR LOVE: What did you recommend at that point?
DR LOVE: What did you recommend at that point?
![]() DR BURSTEIN: She seemed to be having a minor response to this regimen, so we suggested a third cycle. It wasn’t clear how much benefit she was receiving from the chemotherapy by itself, so we began discussing other ways we might treat this particular variant of breast cancer, including clinical trials.
DR BURSTEIN: She seemed to be having a minor response to this regimen, so we suggested a third cycle. It wasn’t clear how much benefit she was receiving from the chemotherapy by itself, so we began discussing other ways we might treat this particular variant of breast cancer, including clinical trials.
We discussed introducing bevacizumab, despite the fact that the data on its use in heavily refractory breast cancer are not so compelling. Kathy Miller’s original study of anthracycline- and taxane-treated patients who received capecitabine with or without bevacizumab did not show a major clinical advantage to adding bevacizumab (Miller 2005).
However, many of us believe that targeting the angiogenesis pathway may offer something different, particularly for patients like this. We’ve all had many patients similar to this one, for whom it seems unlikely chemotherapy itself will turn the tide, and the temptation arises to consider agents like bevacizumab.
Also, we are increasingly developing and participating in specific clinical trials for this type of breast cancer. At our institute, we have several Phase II trials specifically focusing on triple-negative tumors, offering either novel therapies or combinations of therapies.
![]() DR LOVE: Assuming this patient is not eligible for or not interested in participating
in a clinical trial, what’s your next step if her disease progresses on the chemotherapy?
DR LOVE: Assuming this patient is not eligible for or not interested in participating
in a clinical trial, what’s your next step if her disease progresses on the chemotherapy?
![]() DR BURSTEIN: She is currently receiving platinum-based therapy, which has been discussed considerably, particularly for triple-negative tumors, but at this time the literature doesn’t tell us whether these patients will routinely fare better with such a regimen. We have a long list of agents that have been studied in advanced breast cancer, including many familiar options such as capecitabine, vinorelbine and gemcitabine. If her disease progresses, we will start reaching for those.
DR BURSTEIN: She is currently receiving platinum-based therapy, which has been discussed considerably, particularly for triple-negative tumors, but at this time the literature doesn’t tell us whether these patients will routinely fare better with such a regimen. We have a long list of agents that have been studied in advanced breast cancer, including many familiar options such as capecitabine, vinorelbine and gemcitabine. If her disease progresses, we will start reaching for those.
![]() DR LOVE: Would you consider bevacizumab?
DR LOVE: Would you consider bevacizumab?
![]() DR BURSTEIN: I would probably try bevacizumab at some point, although the magnitude of benefit for a case like this is not clear. I would consider offering it with capecitabine, based on the safety data with that combination. George Sledge’s Phase II, first-line trial, the XCaliBr study, demonstrated a reasonable response rate with bevacizumab and capecitabine and confirmed the safety experience previously reported (Sledge 2007; Miller 2006).
DR BURSTEIN: I would probably try bevacizumab at some point, although the magnitude of benefit for a case like this is not clear. I would consider offering it with capecitabine, based on the safety data with that combination. George Sledge’s Phase II, first-line trial, the XCaliBr study, demonstrated a reasonable response rate with bevacizumab and capecitabine and confirmed the safety experience previously reported (Sledge 2007; Miller 2006).
Track 9

![]() DR O’SHAUGHNESSY: The lobular invasive cancer in the gastrointestinal (GI) tract was discovered on routine colonoscopy. The patient was asymptomatic, but when we checked her CA27.29, it was more than 1,000 U/mL.
DR O’SHAUGHNESSY: The lobular invasive cancer in the gastrointestinal (GI) tract was discovered on routine colonoscopy. The patient was asymptomatic, but when we checked her CA27.29, it was more than 1,000 U/mL.
We performed an extensive evaluation with every imaging study known to mankind, including bilateral marrow biopsies, but found nothing. I was surprised, considering that she had 10 positive nodes at baseline. Yet every time we checked her CA27.29, it was clearly rising in the face of adjuvant anastrozole.
![]() DR LOVE: Hal, what is the association between invasive lobular cancer and the GI tract?
DR LOVE: Hal, what is the association between invasive lobular cancer and the GI tract?
![]() DR BURSTEIN: A clinical association has been well documented. Lobular tumors seem to have slightly different tissue predilections than ductal carcinomas when it comes to the initial site of metastasis. They are almost always ER-positive and almost always HER2-negative. In metastatic disease, they spread to visceral sites less frequently, as initial manifestations, and will more likely metastasize
to bone or cirrhosal surfaces, such as the pleura or intra-abdominal cavity.
DR BURSTEIN: A clinical association has been well documented. Lobular tumors seem to have slightly different tissue predilections than ductal carcinomas when it comes to the initial site of metastasis. They are almost always ER-positive and almost always HER2-negative. In metastatic disease, they spread to visceral sites less frequently, as initial manifestations, and will more likely metastasize
to bone or cirrhosal surfaces, such as the pleura or intra-abdominal cavity.
![]() DR LOVE: Joyce, how did the patient fare on anastrozole?
DR LOVE: Joyce, how did the patient fare on anastrozole?
![]() DR O’SHAUGHNESSY: She was feeling great, so we continued the anastrozole. A year later, her GI tract was rescoped and nothing was found. She received anastrozole for another two or three years, and when we checked her CA27.29 every two or three months, it continued to rise another 500 points each time, but still we found nothing.
DR O’SHAUGHNESSY: She was feeling great, so we continued the anastrozole. A year later, her GI tract was rescoped and nothing was found. She received anastrozole for another two or three years, and when we checked her CA27.29 every two or three months, it continued to rise another 500 points each time, but still we found nothing.
I was concerned about the abdominal cavity because it’s a common site for metastases, and I watched for early evidence of hydronephrosis. One particular abdominal CT scan revealed perinephric stranding, so I took that as a sign of trouble and switched her to exemestane.
Her tumor marker did not come down, and she began losing a little weight. I then switched her to megestrol acetate, and she responded. For the first time, her marker began going down, and she gained a few pounds, which was good.
However, after six to nine months her disease began to progress — she became a little anemic and began losing weight again. Considering the documented disease in her colon and the perinephric findings, I was thinking “gut” and placed her on fluoxymesterone.
![]() DR LOVE: How did this patient respond to the fluoxymesterone?
DR LOVE: How did this patient respond to the fluoxymesterone?
![]() DR O’SHAUGHNESSY: She had a fantastic response to fluoxymesterone. I increased the dose to 10 milligrams QID, and month after month her marker went down. It’s currently around 1,200 to 1,500, but it had been as high as 5,000 or 6,000 U/mL.
DR O’SHAUGHNESSY: She had a fantastic response to fluoxymesterone. I increased the dose to 10 milligrams QID, and month after month her marker went down. It’s currently around 1,200 to 1,500, but it had been as high as 5,000 or 6,000 U/mL.
![]() DR LOVE: Has she had any problems on fluoxymesterone?
DR LOVE: Has she had any problems on fluoxymesterone?
![]() DR O’SHAUGHNESSY: She told me she noticed more hair on her legs, but nothing major. I use fluoxymesterone because approximately 80 to 90 percent of ER-positive breast cancer cases have the androgen receptors. It’s one of my “go-to” drugs with lobular breast cancer, and I’ve been impressed with it.
DR O’SHAUGHNESSY: She told me she noticed more hair on her legs, but nothing major. I use fluoxymesterone because approximately 80 to 90 percent of ER-positive breast cancer cases have the androgen receptors. It’s one of my “go-to” drugs with lobular breast cancer, and I’ve been impressed with it.
Lobular cancer is common in the metastatic setting. Even though it accounts for only 15 percent of adjuvant cases because it is not particularly responsive to chemotherapy or endocrine therapy, it accounts for one third of my metastatic practice.
![]() DR BURSTEIN: This is an interesting case, but I’m not sure the literature supports the idea that lobular cases are more resistant to the standard hormone therapies. Historically it’s been said that size for size, node for node, lobular and ductal breast cancer fare comparably if they are ER-positive.
DR BURSTEIN: This is an interesting case, but I’m not sure the literature supports the idea that lobular cases are more resistant to the standard hormone therapies. Historically it’s been said that size for size, node for node, lobular and ductal breast cancer fare comparably if they are ER-positive.
Well, Chuck Vogel used to say that he had a dozen different kinds of endocrine therapy, and I give Joyce kudos for thinking outside of the box and reaching for some older drugs. I don’t believe our fellows have ever seen Halotestin used.
It’s hard to say exactly what’s going on in this case. It’s unusual in that the markers have been so markedly elevated without more measurable disease. The case has many interesting parts, and I’m glad the patient is doing well.
Tracks 13-16

![]() DR BURSTEIN: We started this patient on bisphosphonate therapy for
her bone lesions, in addition to combined endocrine therapy consisting of ovarian suppression and tamoxifen. She experienced an excellent period of tumor control, and at some point she underwent an oophorectomy and continued on tamoxifen.
DR BURSTEIN: We started this patient on bisphosphonate therapy for
her bone lesions, in addition to combined endocrine therapy consisting of ovarian suppression and tamoxifen. She experienced an excellent period of tumor control, and at some point she underwent an oophorectomy and continued on tamoxifen.
Eventually she experienced progression in the bone, so we switched her to an aromatase inhibitor, which she received for eight or nine months. She began having more bone symptoms, and her bone scan suggested possible evolution of her disease. We restaged the cancer but did not find significant visceral disease.
Then the questions arose as to whether we should continue the endocrine therapy or whether we should try chemotherapy, which she hadn’t received.
My colleague at Mass General, Paul Goss, holds this interesting concept involving withdrawal of the aromatase inhibitor, and he has a trial for that. This patient is a bit of an eccentric character, and although she didn’t want to participate in the study, she did want to take a break from all therapy.
We stopped her aromatase inhibitor, and astonishingly she’s doing fine. It’s only been approximately four months, but her disease appears to be stable. Every time she comes into the clinic, I tell her I want to do something, but she’s content to do nothing.
![]() DR LOVE: If she agreed to further therapy, what would you use at this point?
DR LOVE: If she agreed to further therapy, what would you use at this point?
![]() DR BURSTEIN: She had been receiving a nonsteroidal aromatase inhibitor, and I believe the choice would be either to switch her to a steroidal aromatase inhibitor, such as exemestane, or to try fulvestrant. We could also consider chemotherapy, but she really does not want chemotherapy.
DR BURSTEIN: She had been receiving a nonsteroidal aromatase inhibitor, and I believe the choice would be either to switch her to a steroidal aromatase inhibitor, such as exemestane, or to try fulvestrant. We could also consider chemotherapy, but she really does not want chemotherapy.
![]() DR LOVE: Bill Gradishar reported on the EFECT study at the San Antonio Breast Cancer Symposium in 2006, which compared fulvestrant to exemestane after progression on nonsteroidal aromatase inhibitor therapy for patients with advanced breast cancer. That pretty well describes this patient’s options. Joyce, what did you think of the EFECT data?
DR LOVE: Bill Gradishar reported on the EFECT study at the San Antonio Breast Cancer Symposium in 2006, which compared fulvestrant to exemestane after progression on nonsteroidal aromatase inhibitor therapy for patients with advanced breast cancer. That pretty well describes this patient’s options. Joyce, what did you think of the EFECT data?
![]() DR O’SHAUGHNESSY: That trial reassured me because it was a large, well-executed, prospective study and it showed that we have some choices for patients like this (Gradishar 2006). She can receive either exemestane or fulvestrant, and each therapy has an approximately 30 to 33 percent clinical benefit rate (2.3).
DR O’SHAUGHNESSY: That trial reassured me because it was a large, well-executed, prospective study and it showed that we have some choices for patients like this (Gradishar 2006). She can receive either exemestane or fulvestrant, and each therapy has an approximately 30 to 33 percent clinical benefit rate (2.3).
![]() DR LOVE: In the study, how long did the tumor need to be stable for the endpoint of clinical benefit?
DR LOVE: In the study, how long did the tumor need to be stable for the endpoint of clinical benefit?
![]() DR BURSTEIN: It varies from trial to trial. In fact, some studies report clinical benefit simply if no progression is evident at the first staging, so you have to read the fine print.
DR BURSTEIN: It varies from trial to trial. In fact, some studies report clinical benefit simply if no progression is evident at the first staging, so you have to read the fine print.
![]() DR O’SHAUGHNESSY: In EFECT, they used six months, and a third of the patients went six months without progression.
DR O’SHAUGHNESSY: In EFECT, they used six months, and a third of the patients went six months without progression.
![]() DR LOVE: Hal, do you believe there might be a place for fulvestrant or
a combination of fulvestrant with an aromatase inhibitor in the adjuvant setting?
DR LOVE: Hal, do you believe there might be a place for fulvestrant or
a combination of fulvestrant with an aromatase inhibitor in the adjuvant setting?
![]() DR BURSTEIN: In the ATAC trial, combining tamoxifen with an aromatase inhibitor did not improve the prognosis. Therefore, I believe combining fulvestrant and an aromatase inhibitor must be studied to determine whether it confers any benefit.
DR BURSTEIN: In the ATAC trial, combining tamoxifen with an aromatase inhibitor did not improve the prognosis. Therefore, I believe combining fulvestrant and an aromatase inhibitor must be studied to determine whether it confers any benefit.
However, the people who conduct a lot of scientific work with fulvestrant maintain that because it leads to substantial downregulation of estrogen receptor and degradation of the receptor, an advantage may exist in combining it with an aromatase inhibitor.
We have sufficient rationale for conducting trials comparing an aromatase inhibitor with or without fulvestrant in either the metastatic setting or the adjuvant setting.
Similarly, interest exists in using fulvestrant as an extended adjuvant treatment, although getting patients to come in monthly for an injection as opposed to taking a pill daily might be a challenge, so we need to see some compelling data before pursuing such a trial.
Tracks 17-18
![]() DR O’SHAUGHNESSY: This patient’s original adjuvant therapy at the time of diagnosis consisted of AC followed by paclitaxel and subsequent tamoxifen. However, after a disease-free interval of three to four years, she experienced bone recurrence. She had already undergone an oophorectomy and received an aromatase inhibitor, in addition to a trial of another endocrine agent, when I saw her in consultation, and her cancer progressed with extensive bone disease.
DR O’SHAUGHNESSY: This patient’s original adjuvant therapy at the time of diagnosis consisted of AC followed by paclitaxel and subsequent tamoxifen. However, after a disease-free interval of three to four years, she experienced bone recurrence. She had already undergone an oophorectomy and received an aromatase inhibitor, in addition to a trial of another endocrine agent, when I saw her in consultation, and her cancer progressed with extensive bone disease.
She was treated with docetaxel and capecitabine and did benefit from that regimen. However, this time it wasn’t one of those unbelievable, multiyear responses that we sometimes see with this particular luminal B biology. When the disease progressed, this time it was in the liver, as is so often the case with luminal B disease. First it metastasizes to the bone, then to the bone and liver.
She then received bevacizumab with vinorelbine and she responded. However, when the disease progressed, her cancer picked up speed, particularly in the liver, and I was becoming nervous. She had already received most of the major agents we have to offer at that point, so in desperation I drew a serum HER2.
This patient originally had HER2-negative disease as determined by FISH. I had seen negative results on a couple of determinations, but her serum sample was strongly HER2-positive.
I have tested the serum for conversion in six to 10 cases over the last few years, and I’ve always been somewhat disappointed. I’ve seen results like 14, 15, maybe 20 at the most, but nothing impressive. However, this patient’s serum HER2 was 75, so I started treating her with gemcitabine, carboplatin and trastuzumab, and she’s showing a major response.
![]() DR LOVE: What happened to her tumor markers?
DR LOVE: What happened to her tumor markers?
![]() DR O’SHAUGHNESSY: Before this regimen, they were skyrocketing. They kept doubling, and they reached the 3,000 range. However, after she began treatment with this combination, they were halving every time I tested. They are now down to around 1,200 and her liver function tests are normalizing, and she’s only received two cycles so far.
DR O’SHAUGHNESSY: Before this regimen, they were skyrocketing. They kept doubling, and they reached the 3,000 range. However, after she began treatment with this combination, they were halving every time I tested. They are now down to around 1,200 and her liver function tests are normalizing, and she’s only received two cycles so far.
![]() DR LOVE: Did she have symptoms from the tumor?
DR LOVE: Did she have symptoms from the tumor?
![]() DR O’SHAUGHNESSY: Yes, she was experiencing significant bone pain and severe fatigue, and these symptoms have improved as well. Also, she has been experiencing some depression. She’s on an SSRI and was sleeping a lot. She’s usually lying down when I see her in the exam room, but the other day she was sitting up.
DR O’SHAUGHNESSY: Yes, she was experiencing significant bone pain and severe fatigue, and these symptoms have improved as well. Also, she has been experiencing some depression. She’s on an SSRI and was sleeping a lot. She’s usually lying down when I see her in the exam room, but the other day she was sitting up.
I’ve seen a couple of elevated serum HER2 levels after progression on bevacizumab. My partner has a similar patient who was also heavily pretreated, but she too responded when she received chemotherapy and trastuzumab.
![]() DR LOVE: Joyce, what do we know about serum HER2, particularly in patients who have tumors that have been labeled HER2-negative?
DR LOVE: Joyce, what do we know about serum HER2, particularly in patients who have tumors that have been labeled HER2-negative?
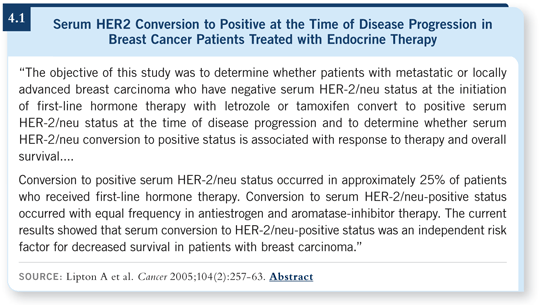
![]() DR O’SHAUGHNESSY: In a paper published in Cancer a year or two ago, Allan Lipton documented that approximately a third of patients with negative serum HER2 at study initiation who had received first-line tamoxifen or letrozole for metastatic or locally advanced breast cancer had a significant elevation of their serum HER2 upon disease progression (Lipton 2005; [4.1]). This was seen in both arms of the study but particularly in the letrozole arm.
DR O’SHAUGHNESSY: In a paper published in Cancer a year or two ago, Allan Lipton documented that approximately a third of patients with negative serum HER2 at study initiation who had received first-line tamoxifen or letrozole for metastatic or locally advanced breast cancer had a significant elevation of their serum HER2 upon disease progression (Lipton 2005; [4.1]). This was seen in both arms of the study but particularly in the letrozole arm.
![]() DR LOVE: Did you think your patient’s disease had transformed?
DR LOVE: Did you think your patient’s disease had transformed?
![]() DR O’SHAUGHNESSY: Yes. A second paper, by Jonathan Uhr at UT Southwestern,
showed clearly in initially HER2-negative disease that approximately 30 percent of the circulating tumor cells were HER2-positive (Meng 2004).
DR O’SHAUGHNESSY: Yes. A second paper, by Jonathan Uhr at UT Southwestern,
showed clearly in initially HER2-negative disease that approximately 30 percent of the circulating tumor cells were HER2-positive (Meng 2004).
![]() DR LOVE: Did you consider a liver biopsy for this patient to document the change in HER2 status?
DR LOVE: Did you consider a liver biopsy for this patient to document the change in HER2 status?
![]() DR O’SHAUGHNESSY: She had recently finished bevacizumab, so I wasn’t too anxious to biopsy her liver, but if she hadn’t been on it, I might have considered
it. However, the serum is different, and one hypothesis is that when a patient experiences progression on bevacizumab, HER2 may be a mechanism of resistance. That hypothesis should be studied.
DR O’SHAUGHNESSY: She had recently finished bevacizumab, so I wasn’t too anxious to biopsy her liver, but if she hadn’t been on it, I might have considered
it. However, the serum is different, and one hypothesis is that when a patient experiences progression on bevacizumab, HER2 may be a mechanism of resistance. That hypothesis should be studied.
![]() DR LOVE: Hal, what do you think about this case?
DR LOVE: Hal, what do you think about this case?
![]() DR BURSTEIN: I see several different issues here. This case underscores the fact that we’re treating advanced breast cancer based on the biology of the disease. Just as we discussed in the first case the need for new agents to treat triple-negative breast cancer, obviously we have several effective agents in the treatment of HER2-positive breast cancer and we need to tailor our therapies for the patient based on that.
DR BURSTEIN: I see several different issues here. This case underscores the fact that we’re treating advanced breast cancer based on the biology of the disease. Just as we discussed in the first case the need for new agents to treat triple-negative breast cancer, obviously we have several effective agents in the treatment of HER2-positive breast cancer and we need to tailor our therapies for the patient based on that.
In question is the nature of this tumor, and several possibilities exist. One is that the initial test results were wrong. We know variations occur in testing quality and reproducibility. A second possibility is that a shift has occurred and a tumor that began life as HER2-negative breast cancer is now emerging as HER2-driven breast cancer.
EDITOR'S NOTE
Peto’s curse
Neil Love, MD
- Select publications
INTERVIEWS
George W Sledge Jr, MD
- Select publications
William J Gradishar, MD
- Select publications
Lee S Schwartzberg, MD
- Select publications
FACULTY TUMOR PANEL
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity