
 |
||||||||

EDITOR’S COMMENT: On this program, breast cancer clinical investigators discuss a number of trials currently under development. Below, find excerpts of these thoughts and several of the trial designs being considered.
WILL THE ADDITION OF A SECOND TARGETED BIOLOGIC THERAPY TO CHEMOTHERAPY/ TRASTUZUMAB IMPROVE ADJUVANT OUTCOMES FOR PATIENTS WITH HER2-POSITIVE TUMORS?
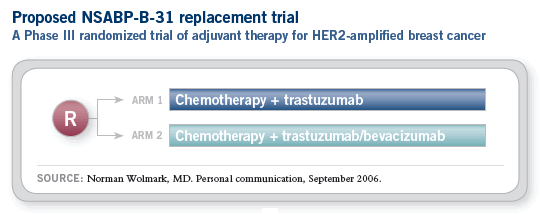
![]() The NSABP is planning a replacement trial for
the NSABP-B-31 study, which will evaluate the
addition of bevacizumab to the B-31 regimen
of AC followed by paclitaxel/trastuzumab with
continued trastuzumab.
The NSABP is planning a replacement trial for
the NSABP-B-31 study, which will evaluate the
addition of bevacizumab to the B-31 regimen
of AC followed by paclitaxel/trastuzumab with
continued trastuzumab.
We’re dealing with the HER2 subset of patients, which is 20 to 25 percent of the total, so it would be foolish to attempt this kind of trial on our own. We would like this to evolve as a global trial, and we’ve been working toward that end in partnership with the BCIRG so we can have every opportunity to be successful in addressing the endpoints with the appropriate power. The preclinical data of the combination of trastuzumab and bevacizumab are interesting. The clinical data are limited to a small subset, but they are exciting.
Our C-08 trial in colon cancer evaluating FOLFOX and bevacizumab just closed with about 2,600 patients accrued in two years. In that study, we have not observed any unanticipated toxicities with bevacizumab in the adjuvant setting.
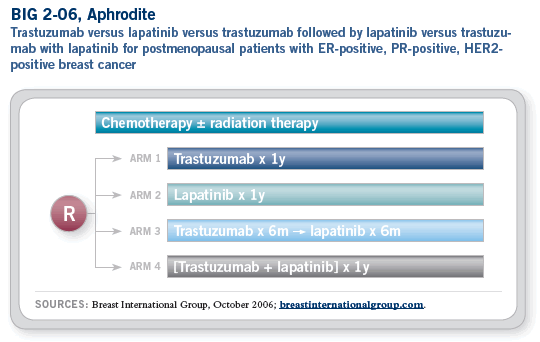
The Aphrodite trial — although it may not actually be called Aphrodite, so the trial formerly known as Aphrodite — is a formidable clinical trial. I believe it addresses a meaningful concept, which is the comparison of trastuzumab to lapatinib to the combination of trastuzumab and lapatinib to the sequence of trastuzumab followed by lapatinib in the adjuvant setting.
We are developing a neoadjuvant trial for patients with HER2-positive disease in which we will compare trastuzumab to lapatinib to the combination of trastuzumab and lapatinib.
Not many patients have received the combination of trastuzumab and lapatinib. The last time I examined the available data, for the approximately 110 or 120 patients who have received this doublet, it appears to be safe as far as the cardiac endpoints are concerned. Beyond that, I don’t believe we have a whole lot of information. I haven’t seen any data that concern me regarding the cardiac effects of lapatinib. I am enthusiastic about the data Chuck Geyer presented at ASCO, and I believe this opens up a new direction for moving forward. However, this needs to be completed in a stepwise, logical way using well-controlled clinical trials.
— Norman Wolmark, MD
![]() The lapatinib data are exciting. Lapatinib is a
small-molecule receptor tyrosine kinase inhibitor
of HER1 and HER2. We have data from a Phase
III trial in refractory metastatic breast cancer
that were presented at the 2006 American
Society of Clinical Oncology meeting by Chuck
Geyer on behalf of an international consortium of
colleagues. This trial randomly assigned patients
who had disease progression on a trastuzumab-based
regimen to receive capecitabine alone, the
FDA standard, or capecitabine with lapatinib in
this anthracycline/taxane-refractory population.
The lapatinib data are exciting. Lapatinib is a
small-molecule receptor tyrosine kinase inhibitor
of HER1 and HER2. We have data from a Phase
III trial in refractory metastatic breast cancer
that were presented at the 2006 American
Society of Clinical Oncology meeting by Chuck
Geyer on behalf of an international consortium of
colleagues. This trial randomly assigned patients
who had disease progression on a trastuzumab-based
regimen to receive capecitabine alone, the
FDA standard, or capecitabine with lapatinib in
this anthracycline/taxane-refractory population.
The results of this trial were strikingly positive in terms of the primary endpoint with, in essence, a doubling of progression-free survival for patients receiving the combination. To date, there’s not been an overall survival advantage in that population, and it may require further maturation of the data to see if that advantage will occur.
Lapatinib is an interesting drug. Based on this trial and previous Phase I and Phase II studies conducted at a number of institutions, lapatinib is a legitimate contender in the metastatic and adjuvant settings for further clinical trials trying to push it, if you will, higher up the food chain to try to improve the curability of HER2-positive breast cancer. I view this as an exciting approach.
— George W Sledge Jr, MD
![]() The US cooperative groups are undertaking
a huge international effort to coordinate the
adjuvant trials for patients with HER2-positive
disease. We’re working with the Europeans, and
the latest design I saw a week ago, which may
already be outdated, has four arms. It has no
mandated chemotherapy regimen per se but
includes a number of choices, and following
the chemotherapy, there will be four arms — a
trastuzumab-alone arm, a lapatanib-alone arm
and arms that have combinations either in
sequence or concurrently.
The US cooperative groups are undertaking
a huge international effort to coordinate the
adjuvant trials for patients with HER2-positive
disease. We’re working with the Europeans, and
the latest design I saw a week ago, which may
already be outdated, has four arms. It has no
mandated chemotherapy regimen per se but
includes a number of choices, and following
the chemotherapy, there will be four arms — a
trastuzumab-alone arm, a lapatanib-alone arm
and arms that have combinations either in
sequence or concurrently.
I don’t believe lapatinib will replace trastuzumab because they have different mechanisms. They will not have complete cross-resistance and have potential for synergy, so lapatinib looks like a terrific drug. Edith Perez is the North American representative for this trial, and there are lots of hurdles but the investigators are putting in a lot of effort. We know that we have down time now with no open trial for these patients, so we are hoping to get this study up and going by the first quarter of 2007.
— Julie R Gralow, MD
HOW CAN NEOADJUVANT TRIALS EXPEDITE THE DEVELOPMENT OF EFFECTIVE SYSTEMIC AGENTS AND REGIMENS?
![]() Right now, the CALGB is aggressively moving in
a new direction. We are developing several clinical
trials in the preoperative setting to address the
global problem we have, which is to develop drugs
in the face of an exciting development — the
falling hazard rates in our clinical trials.
Right now, the CALGB is aggressively moving in
a new direction. We are developing several clinical
trials in the preoperative setting to address the
global problem we have, which is to develop drugs
in the face of an exciting development — the
falling hazard rates in our clinical trials.
For example, my understanding is that the adjuvant trial ECOG-E1199, which compared paclitaxel to docetaxel administered every three weeks versus weekly, never reached its target event number that would have generated its first report. The reasons for that are complex, but mostly it’s just that we’re doing better than we ever did before.
Going forward, that low event rate is going to continue to be a challenge for us. For example, among patients with HER2-positive disease being treated with trastuzumab and ER-positive disease being treated over the long haul with hormone therapy, the event rates are going to be even lower than they’ve been. This doesn’t mean that we don’t need better therapy — we do — but proving that we have better therapy will require us to focus our efforts.
Our hope is that in-breast activity will serve as a surrogate for overall benefit and give us a lead on how to proceed. So for HER2-positive disease, we’re developing a clinical trial testing lapatinib with chemotherapy, and for HER2-normal disease, we’re developing a clinical trial testing bevacizumab. We hope to see a lead that one approach is clearly more active in the preoperative setting or that one approach is sometimes associated with specific biological changes that predict lack of benefit. We need to explore these prospectively before we get to large adjuvant trials.
— Clifford Hudis, MD
![]() We previously planned on working with ACOSOG
in initiating a neoadjuvant trial for patients with
HER2-positive disease that would examine the
Buzdar regimen — paclitaxel
We previously planned on working with ACOSOG
in initiating a neoadjuvant trial for patients with
HER2-positive disease that would examine the
Buzdar regimen — paclitaxel ![]() FEC with trastuzumab
— compared to a more standard trastuzumab-containing regimen in order to confirm the
robust pathologic complete response rate reported
for that small subset of patients. For various
reasons, this trial was not initiated as a partnership
between our groups, but I believe ACOSOG
will move it forward.
FEC with trastuzumab
— compared to a more standard trastuzumab-containing regimen in order to confirm the
robust pathologic complete response rate reported
for that small subset of patients. For various
reasons, this trial was not initiated as a partnership
between our groups, but I believe ACOSOG
will move it forward.
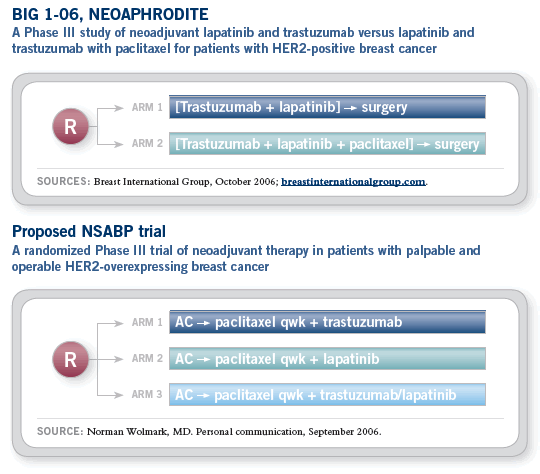
The NSABP is now in the process of developing a
neoadjuvant trial for patients with HER2-positive
disease, which will compare trastuzumab versus
lapatinib versus the combination administered
with AC ![]() paclitaxel. The trial will be powered to
evaluate pathologic complete response rate and
for evolving a molecular taxonomy.
paclitaxel. The trial will be powered to
evaluate pathologic complete response rate and
for evolving a molecular taxonomy.
— Norman Wolmark, MD
WILL THE USE OF BEVACIZUMAB IN THE ADJUVANT SETTING IMPROVE THE OUTCOMES OF PATIENTS WITH HER2-NEGATIVE TUMORS?
![]() The Intergroup trial E2100 randomly assigned
patients in the front-line setting to receive paclitaxel
with or without bevacizumab for the treatment
of metastatic breast cancer. The data from
that trial, with now increasingly mature follow-up,
suggest a striking improvement in progression-free
survival for patients who received the combination
therapy.
The Intergroup trial E2100 randomly assigned
patients in the front-line setting to receive paclitaxel
with or without bevacizumab for the treatment
of metastatic breast cancer. The data from
that trial, with now increasingly mature follow-up,
suggest a striking improvement in progression-free
survival for patients who received the combination
therapy.
In metastatic breast cancer, this represents the first important proof-of-concept study, suggesting that anti-VEGF targeted therapy in particular and anti-angiogenic therapy in general could have a real benefit for patients with advanced disease.
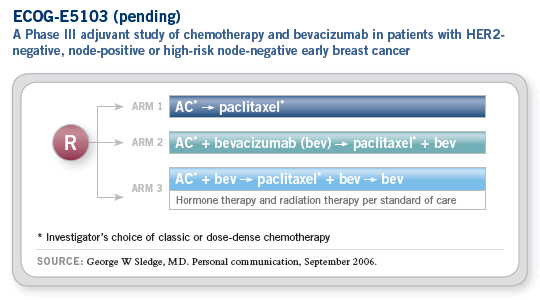
Now, as always in breast cancer, when we have a positive result for patients who are, in large part, incurable, we like to move that therapy as quickly as possible into a setting where we might be able to improve the curability of the disease. This has led to the development of the Breast Cancer Intergroup trial E5103.
This study will randomly assign patients with lymph node-positive breast cancer to one of three arms. The first arm, the backbone chemotherapy arm, will be doxorubicin and cyclophosphamide followed by paclitaxel.
It is interesting that, to improve accrual in the trial and because many physicians still have biases one way or the other, we are allowing physicians to choose whether patients receive the chemotherapy on a more classic every three-week basis or on a dose-dense schedule.
Patients in the second and third arms of the trial will receive bevacizumab concurrent with the AC and the paclitaxel. The difference will be the duration of bevacizumab therapy. In the second arm, patients will receive bevacizumab only during the course of chemotherapy, whereas in the third arm patients will continue bevacizumab for a total duration of one year.
The duration question is one that we haven’t answered very well with other biologics. Trastuzumab springs to mind as the one where eight or nine years after the drug came on the market, we’re still wrestling with how long we should administer it. In the E5103 trial, we’ll be able to answer this question from the get-go with bevacizumab in an appropriate adjuvant population.
This study is also interesting from a biological standpoint. We don’t have a good sense of the mechanism of action of bevacizumab. If one believes there is synergy between chemotherapeutics and anti-angiogenics against endothelial cells, which preclinical data suggest, or that bevacizumab is altering the ability of chemotherapy to get into a tumor, based on Rakesh Jain’s observations, then a great deal of the effect might be a magnifier effect and, therefore, we might see the greatest benefit with a short duration of therapy.
However, it might be that bevacizumab benefits patients via chronic suppression of new blood vessel formation and, therefore, longer duration might be important. I believe the second and third arms will give us an answer to that important question.
I’m incredibly excited about this trial. I view it as the culmination of a decade’s work with anti-angiogenic therapy in breast cancer. If we consider the initial pivotal metastatic trial for trastuzumab in breast cancer and compare it to E2100, with regard to hazard ratios and progression- free survival improvement, E2100 looks as positive as the pivotal trastuzumab trial did a decade ago.
If one can extrapolate from the metastatic setting to the adjuvant setting, then we might see a striking result in the adjuvant setting for a much broader population of patients. With regard to the issue of arterial events, if one examines all of the metastatic trial data with bevacizumab, one sees a modest increase in these events, which raises concerns for the long-term risk of cerebrovascular events and myocardial infarction. However, I suspect that in an adjuvant setting this would be less of a problem. Adjuvant patients are, on average, perhaps a half decade younger than our patients with metastatic disease, and they tend to have fewer comorbidities.
We’re more aware of the potential for toxicities such as hypertension and I suspect we’ll be increasingly better at controlling those, so I’m cautiously hopeful that this will not be a big issue down the road.
— George W Sledge Jr, MD
HOW CAN THE BENEFITS OF ENDOCRINE THERAPY BE IMPROVED BY EXPLOITING GROWTH FACTOR RECEPTOR-ESTROGEN RECEPTOR CROSS-TALK?
![]() Hal Burstein has a clinical trial that’s just
opening evaluating fulvestrant with lapatinib. It’s
not restricted to HER2-positive disease because
there are reasons to argue that HER1 and HER2
inhibition will be additive to fulvestrant.
Hal Burstein has a clinical trial that’s just
opening evaluating fulvestrant with lapatinib. It’s
not restricted to HER2-positive disease because
there are reasons to argue that HER1 and HER2
inhibition will be additive to fulvestrant.
The CALGB is also considering a series of EGFR inhibitors in collaboration with investigators around the United States. As part of that effort, we have begun to evaluate, in collaboration with John Park and Hope Rugo at UCSF, the predictive value of both circulating tumor cells and circulating endothelial cells. From that collaboration in particular, we have the clinical trial that proved the safety of combining bevacizumab with letrozole, and that has led to a randomized trial in the CALGB. Preclinical evidence argues that profound estrogen deprivation may have an antiangiogenic effect and that bevacizumab could add to that.
— Clifford Hudis, MD
I am the principal investigator for a study that the CALGB has just activated for patients with hormone receptor-positive breast cancer whose tumors have some degree of HER2 overexpression — 1+, 2+ or 3+ by IHC but FISH-negative, and they will be randomly assigned to fulvestrant alone versus fulvestrant with the dual kinase inhibitor lapatinib as treatment for metastatic breast cancer. The question we are asking is whether or not inhibition of EGFR and HER2 can potentiate the effects of antiestrogen therapy. Many laboratory models — most notably those of Kent Osborne at Baylor — have suggested that if you interfere with the two pathways at once, you can make the antiestrogen effect more potent.
We are going to critically evaluate that in the CALGB. We lowered the bar for HER2 expression, compared to just the strongly positives, for three reasons. One is that for patients who have aromatase inhibitor resistance, which is what is expected in this trial, there is some suggestion that you might get upregulation of HER2. The other is that the threshold for HER2 expression for a benefit from lapatinib has not yet been established, and the third is that because lapatinib has this EGFR inhibition potential, we wanted to see if some of that might also be in play. So that study is being activated around the country, and I hope it will accrue handsomely.
— Harold J Burstein, MD, PhD
![]() Our group’s main focus is to understand how
tumors become resistant to hormone therapy,
and what we’ve discovered over the years — as
well as others — is the relationship between
growth factor receptors such as HER2 and the
estrogen receptor pathway. In a sense, these
pathways “talk” to each other and amplify the
signals from each of the different receptors.
Our group’s main focus is to understand how
tumors become resistant to hormone therapy,
and what we’ve discovered over the years — as
well as others — is the relationship between
growth factor receptors such as HER2 and the
estrogen receptor pathway. In a sense, these
pathways “talk” to each other and amplify the
signals from each of the different receptors.
We have data from our laboratory studies — that are beginning to be supported by results from clinical trials — indicating that one of the ways tumors can become resistant to endocrine therapies like tamoxifen and aromatase inhibitors is through cross-talk between growth factor pathways and the estrogen receptor pathway. If that is the case, then it makes sense to try to block multiple pathways simultaneously in the appropriate tumor to achieve maximum benefit. If a tumor that expresses the estrogen receptor also overexpresses HER2, our data suggest that in order to obtain the optimal benefit from those therapies, you need to target both receptors by combining targeted therapies.
— C Kent Osborne, MD
![]() We’re now learning that if you shut down one
growth factor receptor pathway, you may be
upregulating another, and that we should be
thinking about multiple-pathway blockades. One
of the more fascinating papers I’ve seen recently
was on lapatinib and was published in the
Proceedings of the National Academy of Sciences by Neil Spector’s group. It showed that if you
treated a HER2-positive, ER-positive cell line
with lapatinib, fairly quickly you got upregulation
of the estrogen receptor.
We’re now learning that if you shut down one
growth factor receptor pathway, you may be
upregulating another, and that we should be
thinking about multiple-pathway blockades. One
of the more fascinating papers I’ve seen recently
was on lapatinib and was published in the
Proceedings of the National Academy of Sciences by Neil Spector’s group. It showed that if you
treated a HER2-positive, ER-positive cell line
with lapatinib, fairly quickly you got upregulation
of the estrogen receptor.
So tumors like to grow, and if they have multiple pathways, we probably need to shut down multiple pathways rather than assuming that in a HER2-positive tumor we only need to target HER2 or in an estrogen receptor-positive tumor we only need to target the estrogen receptor. It’s pretty clear to me that we will need to treat multiple growth factor pathways.
— George W Sledge Jr, MD
HOW CAN GENOMIC MARKERS BE BETTER USED AS PREDICTORS OF RESPONSE TO CHEMOTHERAPY?
![]() The concept of trying to individualize the
treatment of cancer is certainly not new, and
it’s something we are all looking forward to. It
seems clear that if you consider where we are in
cancer care that diagnostics are going to be the
key to unlocking not only more effective use of
our current therapies but also acceleration of the
development of new therapies.
The concept of trying to individualize the
treatment of cancer is certainly not new, and
it’s something we are all looking forward to. It
seems clear that if you consider where we are in
cancer care that diagnostics are going to be the
key to unlocking not only more effective use of
our current therapies but also acceleration of the
development of new therapies.
I think the light bulb went on for everyone with the story of trastuzumab. There is no doubt that, if we had conducted those pivotal trials without a molecular diagnostic test, trastuzumab would have failed. And so, with the advancement of that technology — the ability to look at one gene at a time — it is now possible to look at many genes at once. Clearly, in order to better treat cancer, we need to know cancer. Gene expression profiling with quality technology, rigor and discipline is now allowing us to see cancer and treat it differently.
The Oncotype DXTM assay is currently appropriate for women with node-negative, estrogen receptor-positive tumors. What about node-positive breast cancer? What about women who have micrometastases? What about estrogen receptor-negative breast cancer? If we think about early breast cancer, what about investigating even earlier in the pathogenesis of the disease? What about DCIS? Can we individualize treatment of DCIS? All of these are exciting questions that we hope to answer.
— Steven Shak, MD
The MINDACT (Microarray In Node-negative Disease may Avoid ChemoTherapy) trial will be performed predominantly in Europe, and will be led by the Breast International Group in collaboration with the EORTC and, like TAILORx, will also target women who have lymph node-negative disease. However, it will also allow women who have estrogen receptor-negative disease and HER2-positive disease, which TAILORx does not. The assay they’ll be using is the Amsterdam 70-gene profile, which is commercially available under the name Mammoprint™ and requires collection of a fresh frozen tissue specimen. The trial design also differs from TAILORx in that the patient’s risk of recurrence will also be estimated by Adjuvant! Online.
The 55 percent or so of the patients who are determined by the Mammoprint assay and the clinical criteria — as estimated by Adjuvant! Online — to be at high risk will receive chemotherapy. The 15 percent of patients who are at low risk by both Mammoprint and clinical criteria will receive endocrine therapy alone. The remainder of the patients, for whom there is discordance between the clinical and genomic criteria — which is estimated to be about 35 percent of all patients — will be randomly assigned to treatment by clinical criteria or treatment by genomic criteria.
— Joseph Sparano, MD
Editor’s Note:
Let’s get this thing done.
Prologue:
The adjuvant trastuzumab clinical research to
practice model
- Select publications
Breast Cancer Clinical Trials:
Neoadjuvant Therapy