
 |
||||||||

COMMENTS FROM BREAST CANCER INVESTIGATORS
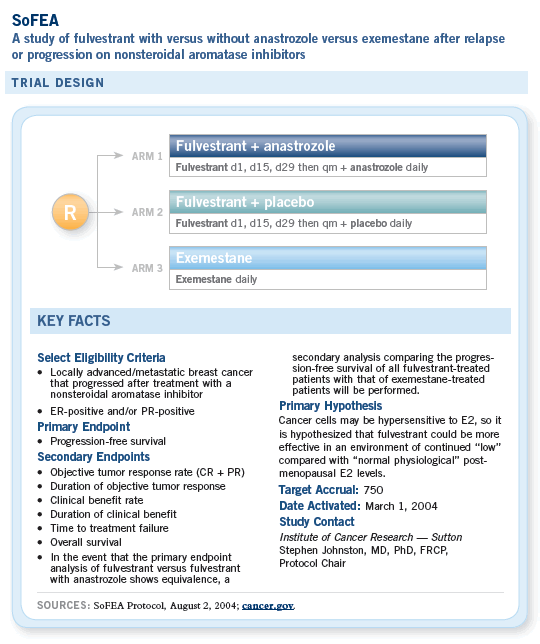
![]() The SoFEA trial is evaluating the use of
endocrine therapy in the metastatic disease
setting, comparing exemestane as a single agent
to fulvestrant to the combination of anastrozole
and fulvestrant.
The SoFEA trial is evaluating the use of
endocrine therapy in the metastatic disease
setting, comparing exemestane as a single agent
to fulvestrant to the combination of anastrozole
and fulvestrant.
The combined therapy arm may be the most interesting one. The rationale behind it is not only removing the ligand for the receptor — which is what the aromatase inhibitor would do by decreasing the amount of circulating estrogen — but also eradicating the actual target, which is the receptor. Answering whether absolute removal of those two targets will result in a better outcome is one of the goals of the study.
— William J Gradishar, MD
![]() In the clinical setting, I think it is a good idea
for patients who are progressing on aromatase
inhibitors to continue with an aromatase inhibitor
and add fulvestrant, but there are no data. I
have done this with a few patients based on two
preclinical studies that have evaluated this: my
own and Angela Brody’s.
In the clinical setting, I think it is a good idea
for patients who are progressing on aromatase
inhibitors to continue with an aromatase inhibitor
and add fulvestrant, but there are no data. I
have done this with a few patients based on two
preclinical studies that have evaluated this: my
own and Angela Brody’s.
Fulvestrant seems to work much better when there’s no estrogen around. Even though postmenopausal women have lower estrogen levels in the blood, their tumors don’t necessarily have lower estrogen levels, and fulvestrant seems to be more effective when estrogen is low.
— C Kent Osborne, MD
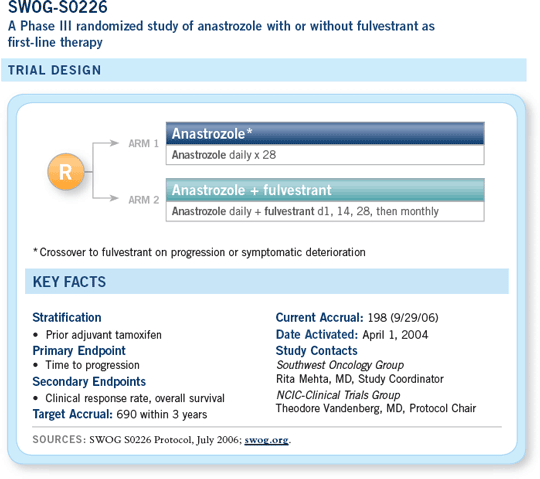
![]() SWOG-S0226 is a randomized, first-line
metastatic study in which all patients receive an
aromatase inhibitor, and half of them will receive
fulvestrant concurrently.
SWOG-S0226 is a randomized, first-line
metastatic study in which all patients receive an
aromatase inhibitor, and half of them will receive
fulvestrant concurrently.
The group that is randomly assigned to receive the aromatase inhibitor alone is then asked to switch to fulvestrant at the time of progression, although we know we can’t force their next-line therapy.
So it’s really a question of an up-front aromatase inhibitor with a selective estrogen receptor down-regulator (SERD), fulvestrant, versus an aromatase inhibitor followed by the SERD.
We’re hoping that we’ll obtain complete estrogen blockade by using this regimen. We know that in the ATAC trial, the anastrozole/tamoxifen combination arm did not appear to be any better than tamoxifen alone and certainly wasn’t going to be the superior arm.
Tamoxifen can have some proestrogenic properties in an otherwise depleted estrogen state. Fulvestrant shouldn’t have these.
It’s a pure antiestrogen and thus is an interesting concept that is different from considering an aromatase inhibitor with or without tamoxifen.
Certainly, preclinical data suggest that this could work. It makes sense, and we have high hopes that it could be better.
— Julie R Gralow, MD
![]() In patients progressing on tamoxifen, tamoxifen
binds the estrogen receptors and may actually
stimulate growth of the tumor — it certainly is no
longer inhibiting it. Treating these patients with
an aromatase inhibitor will be ineffective until
all the tamoxifen is gone, which takes a couple
of months.
In patients progressing on tamoxifen, tamoxifen
binds the estrogen receptors and may actually
stimulate growth of the tumor — it certainly is no
longer inhibiting it. Treating these patients with
an aromatase inhibitor will be ineffective until
all the tamoxifen is gone, which takes a couple
of months.
Fulvestrant, on the other hand, competes with tamoxifen for binding, thus the response may be quicker with fulvestrant than with an aromatase inhibitor in that setting.
— C Kent Osborne, MD
![]() In cell culture, when MCF7 cells are depleted
of estradiol, they become extremely sensitive to
low levels of estrogen. The cell line can be inhibited
if fulvestrant is then titrated into that long-term
estrogen-deprived cell line.
The rationale behind the SoFEA study is that the
development of resistance to aromatase inhibitors
may result from an increased sensitivity of breast
cancer cells to very low levels of estradiol.
In cell culture, when MCF7 cells are depleted
of estradiol, they become extremely sensitive to
low levels of estrogen. The cell line can be inhibited
if fulvestrant is then titrated into that long-term
estrogen-deprived cell line.
The rationale behind the SoFEA study is that the
development of resistance to aromatase inhibitors
may result from an increased sensitivity of breast
cancer cells to very low levels of estradiol.
Fulvestrant competes with estradiol for the estrogen receptor on a one-to-one basis so that upon progression while on the aromatase inhibitor, the addition of fulvestrant to the aromatase inhibitor might result in a better blocking effect.
I hope the SoFEA trial will show that there is improvement from the combination of fulvestrant and an aromatase inhibitor.
This will be an interesting study, not only because it will tell us what to do in second- or third-line therapy but because it will also tell us about mechanisms of action and whether they are important in breast cancer.
— John F R Robertson, MD
COMMENTS FROM BREAST CANCER INVESTIGATORS
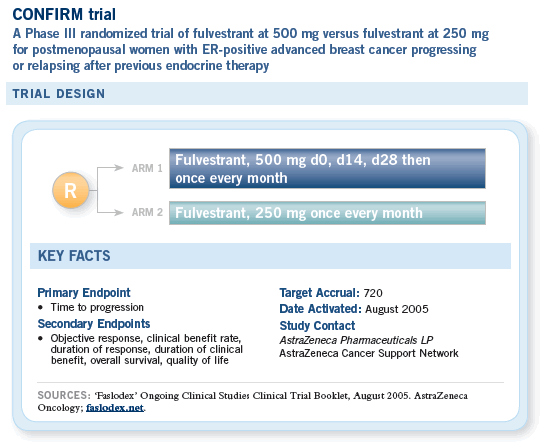
![]() An important issue is whether fulvestrant
at 250 mg is optimal, even though that’s the
approved dose. Some of the data, including
preclinical data generated by Kent Osborne and
others, suggest that this dose is on the low end
of the curve where you might expect the optimal
response rate.
An important issue is whether fulvestrant
at 250 mg is optimal, even though that’s the
approved dose. Some of the data, including
preclinical data generated by Kent Osborne and
others, suggest that this dose is on the low end
of the curve where you might expect the optimal
response rate.
Although we may be able to increase the dose, administering 250 mg in each buttock, doing that too frequently becomes prohibitive, and patients may not tolerate it.
Some strategies have evaluated quickly increasing serum levels of fulvestrant, and those strategies have included administering loading doses of 500 mg and then, within two weeks, administering another 250 mg and then proceeding to the monthly schedule.
Those strategies are based on mathematical modeling that have shown an ability to achieve steady-state levels much quicker and, consequently, achieve a biologically relevant dose of drug circulating in a given patient much faster.
— William J Gradishar, MD
![]() We expected fulvestrant to be superior to
tamoxifen, but in the first-line setting it proved
to be similar, not better.
We expected fulvestrant to be superior to
tamoxifen, but in the first-line setting it proved
to be similar, not better.
That’s peculiar because second-line trials show fulvestrant to be equal to or better than aromatase inhibitors, and aromatase inhibitors have been shown to be superior to tamoxifen.
It may be that we’re just not dosing fulvestrant correctly. We know from the randomized trial that half of the currently recommended dose is insufficient, and we know it takes three to six treatments to achieve steady state blood levels with fulvestrant, so perhaps a higher dose or a loading dose (or both) is required. These options are being investigated.
— C Kent Osborne, MD
![]() Fulvestrant at 250 mg is an effective dose, as
demonstrated by the clinical trials. It is as effective
as anastrozole as second-line therapy and
equivalent to tamoxifen as first-line therapy in
postmenopausal women.
Fulvestrant at 250 mg is an effective dose, as
demonstrated by the clinical trials. It is as effective
as anastrozole as second-line therapy and
equivalent to tamoxifen as first-line therapy in
postmenopausal women.
In premenopausal women, data suggest that 250 mg of fulvestrant is not effective at down-regulating the estrogen receptor.
This raises questions about whether a 250-mg dose of fulvestrant leads to complete down-regulation of the estrogen receptor in postmenopausal women. Could a higher dose of fulvestrant achieve more?
Two strategies exist to increase the dose of fulvestrant. The first is a loading dose sequence. The second is the administration of a higher dose of fulvestrant.
For example, instead of administering one 5-mL injection every month in one buttock, one might administer one 5-mL injection in each buttock, for a total of 500 mg. Future studies are needed to determine the dose-response curve for fulvestrant.
— John F R Robertson, MD
![]() I believe the trials of fulvestrant underestimate
the efficacy of this agent.
I believe the trials of fulvestrant underestimate
the efficacy of this agent.
The dosing schedule used was probably too low because by the time steady state was reached, many patients were off study, presumably because of progression. In my group, we administer loading doses of 500 mg of fulvestrant, followed by 500 mg two weeks later and then 250 mg monthly.
The pharmacokinetics of fulvestrant suggest a loading dose would be beneficial, so it concerns me that the comparison of fulvestrant to anastrozole in a tamoxifen-resistant population might not have revealed the true efficacy of fulvestrant. It showed fulvestrant to be at least as effective as anastrozole, but I expected it to be superior.
We may need to repeat some of these studies with a more appropriate dosing schedule.
— Gabriel N Hortobagyi, MD
![]() An earlier study by John Robertson showed no
biological effect when using a standard dose of
250 mg in premenopausal women.
An earlier study by John Robertson showed no
biological effect when using a standard dose of
250 mg in premenopausal women.
We subsequently performed a study in premenopausal women using a 750-mg dose given in three 250-mg injections, which was remarkably tolerated by patients.
Side effects were all Grade I — some headaches and occasional flushes. No significant reactions occurred at the injection site, and patients had transient discomfort.
We saw much greater activity in the tumor with a 750-mg dose than we did with the 250-mg dose. A significant reduction in proliferation occurred, and as in postmenopausal women at the 250-mg dose, the estrogen and progesterone receptors decreased.
Still, there is a possible need for more than 750 mg, and I am interested in looking at a 1-g loading dose, then 500 mg at regular intervals for it to be effective in premenopausal women.
— Michael J Dixon, MD
![]() I am a little disquieted by the fact that it
can take three to five months to reach a steady
state with fulvestrant. A patient with rapidly
progressing disease may not benefit from fulvestrant,
but fortunately most women with hormone-responsive
breast cancer have relatively indolent
disease.
I am a little disquieted by the fact that it
can take three to five months to reach a steady
state with fulvestrant. A patient with rapidly
progressing disease may not benefit from fulvestrant,
but fortunately most women with hormone-responsive
breast cancer have relatively indolent
disease.
I’m interested in the clinical trial in which they are loading fulvestrant at 500 mg every two weeks for a couple of doses and then reducing it to 250 mg monthly. That makes sense to me.
— Joyce O’Shaughnessy, MD
![]() At MD Anderson, we use a loading dose of
fulvestrant. We administer 500 mg on day one,
250 mg on day 15 and day 29 and then monthly.
Many of the key investigators in the early development
of the drug believe it is important to
attain steady state, but there are no randomized
data for the loading approach.
At MD Anderson, we use a loading dose of
fulvestrant. We administer 500 mg on day one,
250 mg on day 15 and day 29 and then monthly.
Many of the key investigators in the early development
of the drug believe it is important to
attain steady state, but there are no randomized
data for the loading approach.
Currently, it is FDA approved at 250 mg monthly and is reimbursed by Medicare at that dose. With all of those caveats, I believe — and I don’t know if this is my bias — the loading approach is reasonable.
Although we think that may be the best dosing schedule, we won’t know unless we do a pharmacokinetic study to show that the doses are equally effective.
— Vicente Valero, MD
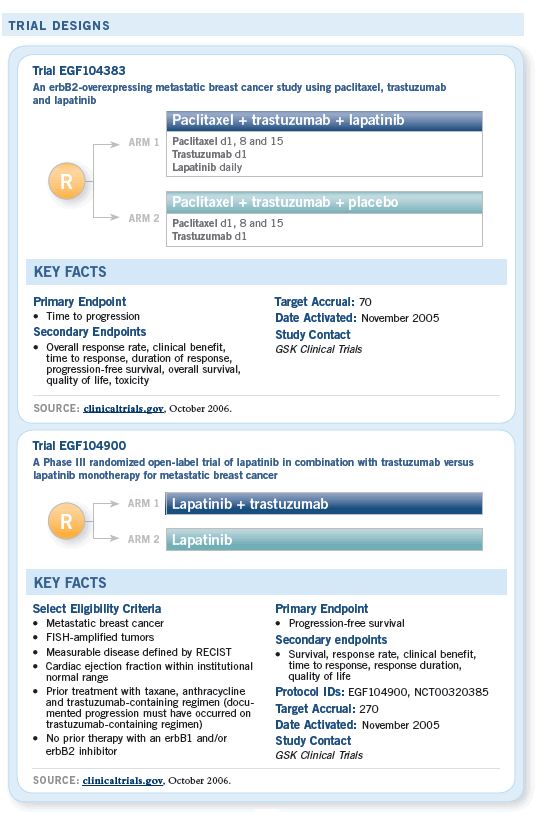
COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() We would like to use drugs that target other
aspects of the HER2 pathway. The current
leading candidate is lapatinib. It’s a dual HER1/HER2 kinase that also inhibits the same target as
trastuzumab, actually, HER2, but it inhibits it in a
different way. It works on the cytoplasmic kinase
domain, which is part of the signaling initiator.
Some early data suggest that when you combine
lapatinib and trastuzumab, you may get a higher
response rate. We know that in early pilot trials,
patients who were previously untreated and had
HER2-positive disease had good response rates
to lapatinib.
We would like to use drugs that target other
aspects of the HER2 pathway. The current
leading candidate is lapatinib. It’s a dual HER1/HER2 kinase that also inhibits the same target as
trastuzumab, actually, HER2, but it inhibits it in a
different way. It works on the cytoplasmic kinase
domain, which is part of the signaling initiator.
Some early data suggest that when you combine
lapatinib and trastuzumab, you may get a higher
response rate. We know that in early pilot trials,
patients who were previously untreated and had
HER2-positive disease had good response rates
to lapatinib.
— Debu Tripathy, MD
![]() Phase I studies of the combination of lapatinib
and trastuzumab revealed that at full doses of
trastuzumab and full doses up to about 1,500
mg of lapatinib, significant fatigue occurred, so
the combination that is tolerable would be with
lapatinib at 1,000 mg. This is an active regimen
in patients who are candidates for trastuzumab,
and it would need to be evaluated.
Phase I studies of the combination of lapatinib
and trastuzumab revealed that at full doses of
trastuzumab and full doses up to about 1,500
mg of lapatinib, significant fatigue occurred, so
the combination that is tolerable would be with
lapatinib at 1,000 mg. This is an active regimen
in patients who are candidates for trastuzumab,
and it would need to be evaluated.
What I find exciting about the results of the lapatinib/capecitabine study is that there is now a second effective drug to shut down HER2 access, which is the main thing one wants to do when treating patients with HER2-positive breast cancer, because like trastuzumab, it seems that lapatinib allows many chemotherapy drugs to work better. So when there is a second drug that shuts down that access in a different way, the question arises whether there are patients in whom one drug would be better than the other, or where the combination would be better. These are issues that need to be studied in the earlier front line, in neoadjuvant and adjuvant settings.
— Charles E Geyer Jr, MD
![]() Trastuzumab and lapatinib work well together,
both in vitro and also in clinical trials, in which
patients will have had multiple trastuzumab-containing
regimens. They have progressive
disease, and then the lapatinib is added to the
trastuzumab. In that case, response rates in the
range of about 27-30 percent are seen.
Trastuzumab and lapatinib work well together,
both in vitro and also in clinical trials, in which
patients will have had multiple trastuzumab-containing
regimens. They have progressive
disease, and then the lapatinib is added to the
trastuzumab. In that case, response rates in the
range of about 27-30 percent are seen.
— Edith A Perez, MD
![]() The small-molecule, erbB2 kinase inhibitors
are an exciting class of compounds that could
be the next to be studied in the adjuvant setting
in HER2-positive disease. Specifically, lapatinib
seems to be the leader, although it’s not a pure
HER2 kinase inhibitor because it also inhibits the
EGF receptor kinase. Be that as it may, it has clear
activity in HER2-positive metastatic disease, and
that’s been presented in Phase II cohorts.
The small-molecule, erbB2 kinase inhibitors
are an exciting class of compounds that could
be the next to be studied in the adjuvant setting
in HER2-positive disease. Specifically, lapatinib
seems to be the leader, although it’s not a pure
HER2 kinase inhibitor because it also inhibits the
EGF receptor kinase. Be that as it may, it has clear
activity in HER2-positive metastatic disease, and
that’s been presented in Phase II cohorts.
We participated in an intriguing study of a combination of trastuzumab and lapatinib, which appears to be promising even in patients with prior treatment failure on trastuzumab. A recent study from South America evaluated single-agent lapatinib in HER2-positive metastatic breast cancer.
Those patients had a response rate of approximately one third, which is similar to what Chuck Vogel presented for single-agent trastuzumab as first-line therapy in patients with HER2-positive metastatic disease, suggesting that lapatinib may have significant activity in this population of patients. So lapatinib is potentially poised to be integrated into the adjuvant setting, either in combination with trastuzumab or following trastuzumab or as a substitute for trastuzumab.
— Mark D Pegram, MD
![]() Studies evaluating lapatinib in combination
with trastuzumab will be important. Preclinical
data suggest there is synergy there. They work
by different mechanisms on the HER2 molecule.
The next Intergroup adjuvant trial will use an
AC
Studies evaluating lapatinib in combination
with trastuzumab will be important. Preclinical
data suggest there is synergy there. They work
by different mechanisms on the HER2 molecule.
The next Intergroup adjuvant trial will use an
AC ![]() paclitaxel chemotherapy regimen as a
backbone. One arm will receive a year of trastuzumab,
one arm will receive both added together
and the third arm, which is under negotiation,
is lapatinib alone without any trastuzumab in
the adjuvant setting. Ethically, I believe it’s an
appropriate arm. We will be reassured by the
head-on comparison data of the two drugs in the
metastatic setting, and if they are looking fairly
equal, it’ll be easier to accrue.
paclitaxel chemotherapy regimen as a
backbone. One arm will receive a year of trastuzumab,
one arm will receive both added together
and the third arm, which is under negotiation,
is lapatinib alone without any trastuzumab in
the adjuvant setting. Ethically, I believe it’s an
appropriate arm. We will be reassured by the
head-on comparison data of the two drugs in the
metastatic setting, and if they are looking fairly
equal, it’ll be easier to accrue.
Up-front single-agent lapatinib has been administered to patients who haven’t received trastuzumab in other parts of the world, where trastuzumab is not readily available. There are historical controls and you take it with all those caveats, but the response rate and time to progression were virtually identical to those seen when trastuzumab was used as a first-line single agent. Chuck Vogel published that years ago.
So, as a single agent, lapatinib has good activity comparable to that previously seen with trastuzumab. We need the comparison, and it might be that it has to be conducted in other parts of the world. We can’t do it here because we have ready access to trastuzumab.
— Julie R Gralow, MD


COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() Interesting data indicate that estrogen may
directly modulate angiogenesis through effects
on endothelial cells in both physiologic and
pathologic conditions. Interesting data also
indicate that antiestrogen therapy blocks VEGF
expression, and estrogen-induced angiogenesis
may be blocked by anti-estrogen therapy.
Interesting data indicate that estrogen may
directly modulate angiogenesis through effects
on endothelial cells in both physiologic and
pathologic conditions. Interesting data also
indicate that antiestrogen therapy blocks VEGF
expression, and estrogen-induced angiogenesis
may be blocked by anti-estrogen therapy.
Rakesh Jain’s group in Boston has observed an androgen-dependent tumor model and shown that castration, interestingly, leads to initial vascular regression, and then there is a second wave of angiogenesis with vascular regrowth in this murine tumor model.
We participated in a Phase II trial combining letrozole with bevacizumab. The hypothesis was that anti-VEGF therapy may overcome this resistance of the second wave of angiogenesis seen with endocrine therapy in animal models and could improve the efficacy of standard hormone therapy in hormone receptor-positive metastatic breast cancer.
Forty-three patients were enrolled in the trial. Patients received bevacizumab at 15 mg/kg every three weeks, as well as letrozole at 2.5 milligrams a day. The combination appears to be well tolerated. The drug-related toxicities were expected and only seen in a small number of patients. The efficacy analysis, which wasn’t the primary goal of this study, was confounded by the long duration of prestudy aromatase inhibitor therapy most patients received, although it did appear that a number of patients might have benefited from the therapy as a hypothesis.
Principal investigators Maura Dickler and Matt Ellis have planned a Phase III study evaluating patients with hormone-positive disease for firstline therapy. Patients will be randomly assigned to endocrine therapy with placebo or endocrine therapy with bevacizumab.
— Hope S Rugo, MD
![]() Both estrogens and progestins induce VEGF
in breast cancer cells through their respective
receptors and via characterized hormone
response elements. Anti-estrogens and antiprogestins
cause some hormone-dependent
tumors to regress; however, some tumor cells
invariably become resistant to anti-hormones and
continue to grow. In certain cases, anti-hormones
can even stimulate tumor growth.
Both estrogens and progestins induce VEGF
in breast cancer cells through their respective
receptors and via characterized hormone
response elements. Anti-estrogens and antiprogestins
cause some hormone-dependent
tumors to regress; however, some tumor cells
invariably become resistant to anti-hormones and
continue to grow. In certain cases, anti-hormones
can even stimulate tumor growth.
It is not known what specifically causes the resistant cells to continue to proliferate, though it has been suggested that growth factors may be involved. Interestingly, clinical studies have shown that tumors with high levels of VEGF fail to respond to hormone therapy or have an early recurrence, suggesting that VEGF production may be responsible for anti-hormone resistance. These studies also re-affirm that VEGF may be responsible for tumor cell proliferation as reported previously. Our recent data indicate that exposure of breast cancer cells to VEGF can override the effects of anti-hormone, suggesting that a treatment regimen of both anti-hormones and anti-angiogenic agents may be better for tumor suppression than a single regimen alone.
— Salman Hyder, PhD. Endocr Relat Cancer
2006;13(3):667-97.
![]() Increased levels of vascular endothelial growth
factor (VEGF) are associated with a poor response
of breast cancer to anti-hormone treatment.
Although VEGF is regarded as an endothelial-specific
growth factor, recent reports have shown
that VEGF can promote proliferation of other cell
types, including breast tumor cells...
Increased levels of vascular endothelial growth
factor (VEGF) are associated with a poor response
of breast cancer to anti-hormone treatment.
Although VEGF is regarded as an endothelial-specific
growth factor, recent reports have shown
that VEGF can promote proliferation of other cell
types, including breast tumor cells...
VEGF stimulates proliferation of VEGFR2-positive tumor cells, promotes survival via the expression and activity of Bcl-2 and overrides the growth-suppressive effects of anti-hormones. This represents a potential explanation for anti-hormone resistance and tumor progression in clinical samples. Thus, it may be useful to use combined modality treatment involving anti-hormones and anti-angiogenic agents to treat breast cancers that express elevated levels of VEGF.
— Yayun Liang, PhD et al. Endocr Relat Cancer
2006;13(3):905-19.
![]() Regulation of soluble VEGFR-1 by estrogen
may represent one of the molecular pathways
responsible for the angiogenic switch during
breast tumorigenesis. Detailed understanding of
the role of estrogen and antiestrogens (ie, tamoxifen)
used in clinical settings to control VEGFR-1
expression may help in the design of new strategies
for preventing resistance to endocrine
therapy and may also help clarify the emerging
role of estrogen in controlling vascularization.
Regulation of soluble VEGFR-1 by estrogen
may represent one of the molecular pathways
responsible for the angiogenic switch during
breast tumorigenesis. Detailed understanding of
the role of estrogen and antiestrogens (ie, tamoxifen)
used in clinical settings to control VEGFR-1
expression may help in the design of new strategies
for preventing resistance to endocrine
therapy and may also help clarify the emerging
role of estrogen in controlling vascularization.
— Michael Elkin, PhD et al. J Natl Cancer Inst
2004;96(11):875-8.
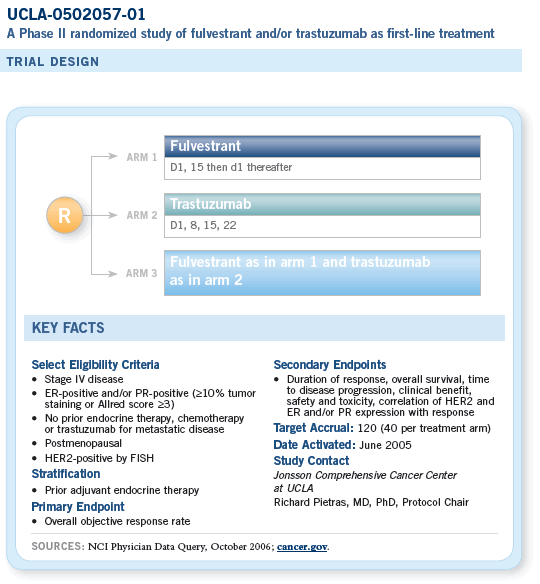
SUPPORTING PROTOCOL INFORMATION
Correlative Science Program
COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() Combining fulvestrant and trastuzumab makes
sense to me, and the reason is that you can see
ligand-independent activation of the estrogen
receptor in HER2-positive breast cells. That is,
the cross talk between HER2 signaling and the
estrogen receptor can activate estrogen-dependent
genes in the absence of estradiol. What
that predicts is that with aromatase inhibitors,
you’ll have an absence of estradiol and no ligand
for the ER, but the ER can still be turned on by
HER2 signaling. So that’s a kind of strike against
aromatase inhibitors. SERMs can actually be
more agonistic after this cross talk mechanism.
How can you tackle such a complex issue? One
idea would be to get rid of the estrogen receptor,
and that’s exactly what fulvestrant does. So it
is appealing from a theoretical point of view to
incorporate HER2-directed therapy with fulvestrant,
and we have a randomized Phase II trial
under way in the metastatic setting, comparing
fulvestrant alone to trastuzumab alone to the
combination.
Combining fulvestrant and trastuzumab makes
sense to me, and the reason is that you can see
ligand-independent activation of the estrogen
receptor in HER2-positive breast cells. That is,
the cross talk between HER2 signaling and the
estrogen receptor can activate estrogen-dependent
genes in the absence of estradiol. What
that predicts is that with aromatase inhibitors,
you’ll have an absence of estradiol and no ligand
for the ER, but the ER can still be turned on by
HER2 signaling. So that’s a kind of strike against
aromatase inhibitors. SERMs can actually be
more agonistic after this cross talk mechanism.
How can you tackle such a complex issue? One
idea would be to get rid of the estrogen receptor,
and that’s exactly what fulvestrant does. So it
is appealing from a theoretical point of view to
incorporate HER2-directed therapy with fulvestrant,
and we have a randomized Phase II trial
under way in the metastatic setting, comparing
fulvestrant alone to trastuzumab alone to the
combination.
I have a number of patients with ER-positive, HER2-positive disease who are on fulvestrant and trastuzumab and are doing well, though they were started on the treatment off protocol because the protocol wasn’t open when they started, and they’re still on it. So I’ve had some nice anecdotal responders on that combination.
— Mark D Pegram, MD
![]() Our group’s main focus is to understand how
tumors become resistant to hormone therapy,
and others have discovered over the years that
there is a relationship between growth factor
receptors, such as HER2 and others, and their
activities and the estrogen receptor pathway.
In a sense, these pathways talk to each other
and amplify the signals coming from each. Data
from our laboratory studies, now supported by
clinical studies, indicate that one of the ways
that tumors become resistant to tamoxifen and
to estrogen-deprivation therapies such as aromatase
inhibitors is from cross talk between growth
factor pathways and the estrogen receptor. If
that were the case, it would make sense to block
both pathways simultaneously in the appropriate
tumor to obtain maximum benefit. For instance,
if a tumor expresses estrogen receptor and
overexpresses HER2, our data suggest that it
would be necessary to target both to achieve
optimal benefit. Using trastuzumab to block
HER2 and leaving the estrogen receptor wide
open would not provide very good results, nor
would much ground be gained by blocking the
estrogen receptor and leaving HER2 wide open,
because they cross talk with each other.
Our group’s main focus is to understand how
tumors become resistant to hormone therapy,
and others have discovered over the years that
there is a relationship between growth factor
receptors, such as HER2 and others, and their
activities and the estrogen receptor pathway.
In a sense, these pathways talk to each other
and amplify the signals coming from each. Data
from our laboratory studies, now supported by
clinical studies, indicate that one of the ways
that tumors become resistant to tamoxifen and
to estrogen-deprivation therapies such as aromatase
inhibitors is from cross talk between growth
factor pathways and the estrogen receptor. If
that were the case, it would make sense to block
both pathways simultaneously in the appropriate
tumor to obtain maximum benefit. For instance,
if a tumor expresses estrogen receptor and
overexpresses HER2, our data suggest that it
would be necessary to target both to achieve
optimal benefit. Using trastuzumab to block
HER2 and leaving the estrogen receptor wide
open would not provide very good results, nor
would much ground be gained by blocking the
estrogen receptor and leaving HER2 wide open,
because they cross talk with each other.
Fulvestrant is a purer antagonist of the estrogen receptor and also induces receptor degradation. It behaves much like an aromatase inhibitor. An aromatase inhibitor deprives the estrogen receptor of its activating ligand, estrogen, whereas fulvestrant blocks and eliminates the estrogen receptor. In experimental models, the end result seems to be the same. Therefore, the mechanisms of resistance to one are identical to the mechanisms of resistance to the other.
In our experimental models, in a tumor that is HER2-positive or that acquires increased expression of HER2 or other growth factors with treatment over time, the increasing activity through the HER2 pathway downregulates the estrogen receptor, and the tumor evolves into one that is estrogen receptor-negative. What is happening there is that over time, the increasing HER2 activity down-regulates the expression of estrogen receptor and progesterone receptor, via mechanisms described by others, resulting in a tumor that is negative for those receptors. Could you recover estrogen receptor expression if the HER2 pathway is blocked? Or are there patients with tumors that start out as ER-negative, HER2-positive that could become ER-positive if HER2 is blocked? Now some increasing clinical information is showing that in some patients, the estrogen receptor comes back after HER2 is blocked with trastuzumab or other drugs. This is an interesting new observation both in the laboratory and clinic, and it may be that some of these ER-negative tumors are really not ER-negative.
— C Kent Osborne, MD
Preclinical investigations using in vitro and xenograft models have led to advances in the understanding of the biology of ER and cross-talk with growth factor signal transduction systems. By degrading the ER, fulvestrant may be less likely than tamoxifen to result in the development of endocrine resistance via elevated growth factor signaling cross-talk. Consequently, there is now considerable interest in exploring combinations of fulvestrant with drugs such as trastuzumab, targeted against HER2, gefitinib, targeted against EGFR, and other agents targeted at inhibiting growth factor signaling. Based on the promising activity of these agents in preclinical investigations, several clinical trials have been initiated to assess the effectiveness of such a combination approach in breast cancer therapy.
— Mitchell Dowsett et al.
Breast Cancer Res Treat 2005;93:S11-18.
![]() The existence of “cross-talk” between various
growth factor receptor signaling pathways and
estrogen receptors is now well-established;
accumulating evidence suggests that estrogen
receptors can become activated and super-sensitized
by a number of different intracellular
kinases, both initially and at the time of relapse.
Estrogen receptors remain an integral part of
signaling even after failure of aromatase inhibitors
or tamoxifen. Therefore, strategies to target
the enhanced expression and pathways that
activate estrogen receptors need to be explored
clinically. Tumor cells are capable of easily
bypassing a single agent inhibitor. Consequently,
clinical trials combining endocrine therapies
and signal transduction inhibitors should be a
high priority. Furthermore, targeting multiple
vital pathways would theoretically have more
antitumor effect and possibly decrease resistance
to each individual agent.
The existence of “cross-talk” between various
growth factor receptor signaling pathways and
estrogen receptors is now well-established;
accumulating evidence suggests that estrogen
receptors can become activated and super-sensitized
by a number of different intracellular
kinases, both initially and at the time of relapse.
Estrogen receptors remain an integral part of
signaling even after failure of aromatase inhibitors
or tamoxifen. Therefore, strategies to target
the enhanced expression and pathways that
activate estrogen receptors need to be explored
clinically. Tumor cells are capable of easily
bypassing a single agent inhibitor. Consequently,
clinical trials combining endocrine therapies
and signal transduction inhibitors should be a
high priority. Furthermore, targeting multiple
vital pathways would theoretically have more
antitumor effect and possibly decrease resistance
to each individual agent.
— Zeina A Nahleh, Abdul-Rahman Jazieh.
Am J Clin Oncol 2005;28:631-3.


COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() Bevacizumab is a very exciting agent, and I
go countercurrent to some of my colleagues, in
that some oncologists take a hard line by saying,
“It didn’t work with capecitabine and I’m only
going to use it in the front line, and I’m not
going to use it out back.” I’ve seen a couple of
absolutely phenomenal responses in women with
far-advanced disease who have failed many prior
forms of chemotherapy.
Bevacizumab is a very exciting agent, and I
go countercurrent to some of my colleagues, in
that some oncologists take a hard line by saying,
“It didn’t work with capecitabine and I’m only
going to use it in the front line, and I’m not
going to use it out back.” I’ve seen a couple of
absolutely phenomenal responses in women with
far-advanced disease who have failed many prior
forms of chemotherapy.
One woman comes to mind. Her tumor was HER2-positive, and she was already being treated with trastuzumab. We elected to treat her with trastuzumab, nab paclitaxel and bevacizumab. She had huge intra-abdominal masses that have virtually disappeared, and she is able to enjoy her life and her family.
Another young woman was treated with a combination of gemcitabine and bevacizumab and had tumor markers in the many thousands. She had a dramatic antitumor response to the combination, and this is third- or fourth-line therapy.
Within the RIBBON 1 trial, we have the ability to use an anthracycline-based combination, a taxane-based combination or capecitabine, and in keeping with my personal philosophy of trying nonalopecic regimens, four of the first six patients I put on the study were treated with capecitabine. All four are undergoing some form of antitumor response, objectively and subjectively. Whether they are receiving bevacizumab or not, we don’t know.
— Charles L Vogel, MD
![]() I believe the results of ECOG-E2100 are
impressive enough that, in the absence of a
contraindication to bevacizumab, I would now
use it in a first-line setting, optimally in combination
with paclitaxel as administered in the
study. I doubt that the interaction is specific
between paclitaxel and bevacizumab, although
when administered with capecitabine in more
advanced disease, bevacizumab seemed to be
less active. That’s probably related to the setting
rather than the drug.
I believe the results of ECOG-E2100 are
impressive enough that, in the absence of a
contraindication to bevacizumab, I would now
use it in a first-line setting, optimally in combination
with paclitaxel as administered in the
study. I doubt that the interaction is specific
between paclitaxel and bevacizumab, although
when administered with capecitabine in more
advanced disease, bevacizumab seemed to be
less active. That’s probably related to the setting
rather than the drug.
— Eric P Winer, MD
![]() In ECOG-E2100 the progression-free survivals
are now approximately a year for the combination
of bevacizumab and paclitaxel. If we saw
progression-free survivals in the same ballpark
in the XCaliBr trial evaluating bevacizumab and
capecitabine as first-line therapy, I believe we’d
all find that very exciting, and it would certainly
suggest that we might be able to combine bevacizumab
successfully with other chemotherapeutic
agents in a more up-front population.
In ECOG-E2100 the progression-free survivals
are now approximately a year for the combination
of bevacizumab and paclitaxel. If we saw
progression-free survivals in the same ballpark
in the XCaliBr trial evaluating bevacizumab and
capecitabine as first-line therapy, I believe we’d
all find that very exciting, and it would certainly
suggest that we might be able to combine bevacizumab
successfully with other chemotherapeutic
agents in a more up-front population.
It becomes important in an era when patients are receiving more and more of their therapy in the adjuvant setting, or more intensive chemotherapy in the adjuvant setting, so that drugs like capecitabine might be a preferential first choice for many patients in the front-line metastatic setting.
— George W Sledge Jr, MD
![]() The bevacizumab story is interesting because,
although I focus on breast cancer, it seems to
work in almost every tumor type in which it has
been studied. It is clearly active in breast cancer.
The E2100 study reported by Kathy Miller
demonstrated the same order of benefit that we
saw combining trastuzumab with chemotherapy
in metastatic disease, and suddenly everyone’s
excited about moving bevacizumab into the
adjuvant setting. Randomized studies are under
way evaluating single agents — gemcitabine,
docetaxel, doxorubicin, capecitabine and nab paclitaxel — with or without bevacizumab to
prove efficacy.
The bevacizumab story is interesting because,
although I focus on breast cancer, it seems to
work in almost every tumor type in which it has
been studied. It is clearly active in breast cancer.
The E2100 study reported by Kathy Miller
demonstrated the same order of benefit that we
saw combining trastuzumab with chemotherapy
in metastatic disease, and suddenly everyone’s
excited about moving bevacizumab into the
adjuvant setting. Randomized studies are under
way evaluating single agents — gemcitabine,
docetaxel, doxorubicin, capecitabine and nab paclitaxel — with or without bevacizumab to
prove efficacy.
— Stephen E Jones, MD
![]() In the metastatic disease trials, the reasons for
focusing on progression-free survival rather than
response rate or overall survival are complex.
First, there is an appreciation that clinical
improvement with response is a relatively soft
endpoint for most patients. Patients would like to
live longer and live free of cancer longer.
In the metastatic disease trials, the reasons for
focusing on progression-free survival rather than
response rate or overall survival are complex.
First, there is an appreciation that clinical
improvement with response is a relatively soft
endpoint for most patients. Patients would like to
live longer and live free of cancer longer.
Second, a theoretical argument exists that newer drugs that target vasculature might not contribute to response as much as they may simply delay progression. So with some of the drugs that are thought to be inhibitors of tumor differentiation or drugs that might slow down angiogenesis, you might see improvement in progression-free survival without a difference in objective response.
For instance, with bevacizumab in the Phase II trials in renal cell cancer, there were hardly any responses, but we did see a dose-dependent difference in time to progression, even though few patients had objective responses. Interestingly, that has not, as yet, been the case with traditional solid tumors in lung, colon and breast studies. The improvement in progression-free survival has been more or less matched by improvements in response rate. What’s lacking in all the bevacizumab studies to date is a predictive marker indicating which patients are likely to benefit and which are not. We don’t have a marker like estrogen receptor or HER2 that would identify patients who are more likely to respond.
— Harold J Burstein, MD, PhD
![]() We have observed that in patients with
metastatic breast cancer, the absence or
continued presence of elevated tumor cells after
therapy made an enormous difference in the time
to tumor progression. I believe more patients will
clear their circulating tumor cells as a consequence
of bevacizumab. Several clinical trials
of bevacizumab are now including correlative
studies of circulating endothelial cells.
We have observed that in patients with
metastatic breast cancer, the absence or
continued presence of elevated tumor cells after
therapy made an enormous difference in the time
to tumor progression. I believe more patients will
clear their circulating tumor cells as a consequence
of bevacizumab. Several clinical trials
of bevacizumab are now including correlative
studies of circulating endothelial cells.
— Daniel Hayes, MD
Editor’s Note:
Let’s get this thing done.
Prologue:
The adjuvant trastuzumab clinical research to
practice model
- Select publications
Breast Cancer Clinical Trials:
Neoadjuvant Therapy