
 |
||||||||
COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() To understand the rationale for this Phase III
trial, you have to understand that when we began
using partial breast irradiation, we selected
patients carefully — patients with small tumors,
clear margins and negative lymph nodes. We
were trying to determine whether this technique
was as efficacious as whole breast irradiation,
but we selected only patients at low risk and,
indeed, the five- and 10-year results with these
low-risk cases have been good.
To understand the rationale for this Phase III
trial, you have to understand that when we began
using partial breast irradiation, we selected
patients carefully — patients with small tumors,
clear margins and negative lymph nodes. We
were trying to determine whether this technique
was as efficacious as whole breast irradiation,
but we selected only patients at low risk and,
indeed, the five- and 10-year results with these
low-risk cases have been good.
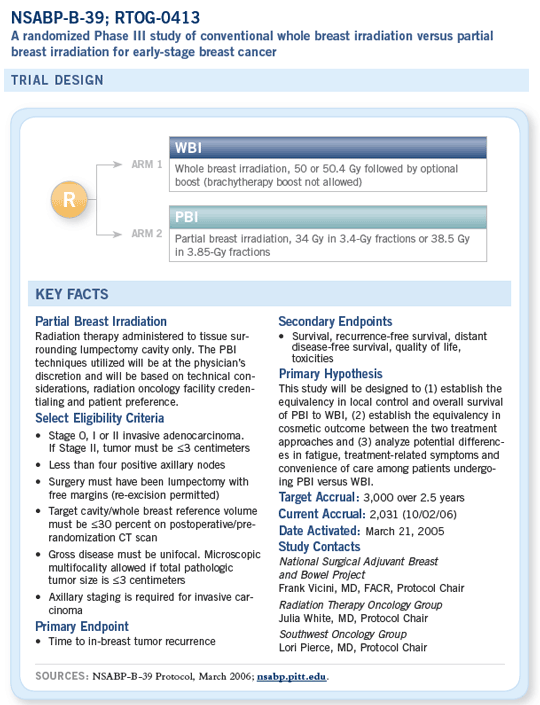
However, with the NSABP-B-39 trial, the eligibility criteria have been loosened significantly. We are treating patients with up to three positive lymph nodes and tumors of up to three centimeters. We’re including multiple types of histologies, not just infiltrating ductal carcinomas. The B-39 trial has been designed to test whether partial breast irradiation could be used for patients at a slightly higher risk or whether it should be restricted to patients at a lower risk.
The three partial breast irradiation techniques used in the trial are brachytherapy with the traditional multiple needles, the MammoSite™ balloon catheter and 3-D conformal external beam radiation therapy. If a patient is interested in participating in the trial, we first do a prerandomization CT scan. The radiation oncologist, with assistance from the surgeon, will examine the lumpectomy cavity on the CT scan to determine whether a patient is a candidate for partial breast irradiation and then, specifically, which partial breast irradiation technique that patient is qualified for from a technical standpoint.
If the patient qualifies for one of the techniques, we let her know. If she agrees to that technique, the patient is then randomly assigned to either whole breast irradiation therapy or that particular partial breast irradiation therapy. If the patient is a candidate for all three partial breast irradiation techniques, then she tells us which one she wants and the randomization is between whole breast irradiation and the technique she’s chosen.
— Frank A Vicini, MD
![]() In our population-based study utilizing SEER
registry data to evaluate patient decision-making
in the selection of mastectomy versus breast-conserving
surgery, we observed significant
concerns about radiation therapy, which were
due to a mixture of fears of radiation toxicity and
concerns about the six weeks of conventional
treatment. Partial breast irradiation has the
opportunity to increase breast conservation rates
particularly for working women, lower-income
women and women in rural areas.
In our population-based study utilizing SEER
registry data to evaluate patient decision-making
in the selection of mastectomy versus breast-conserving
surgery, we observed significant
concerns about radiation therapy, which were
due to a mixture of fears of radiation toxicity and
concerns about the six weeks of conventional
treatment. Partial breast irradiation has the
opportunity to increase breast conservation rates
particularly for working women, lower-income
women and women in rural areas.
The results of the joint NSABP/RTOG trial are going to be important because even though the rationale behind partial breast irradiation is sound, most local recurrences occur in the area of the primary tumor, and whole breast irradiation does not prevent the long-term development of second primary cancers. We have to see whether or not local control is as good as with whole breast irradiation, particularly with cosmetic outcome, and whether shorter-term treatments at higher doses result in greater rates of fibrosis, retraction and fat necrosis.
— Monica Morrow, MD
SUPPORTING PROTOCOL INFORMATION
Background Information
Can an acceptable outcome be achieved with radiotherapy (RT) delivered only to the region of the tumor bed? If this were the case, radiation therapy could be delivered in 1 to 2 weeks, thus significantly shortening treatment time and potentially reducing health care costs. A shortened treatment schedule would decrease the burden of care for patients undergoing breast conserving therapy (BCT), thus making available the conservation option for more women. By reducing the length of time required to deliver RT, the logistical problems associated with integrating local and systemic therapies would also be eliminated.
Additionally, toxicity to adjacent normal structures (ie, heart, underlying chest wall, contralateral breast) should be reduced significantly by decreasing the volume of irradiated tissue.
Several recent meta-analyses on the use of RT for breast cancer patients clearly document a reduction in cancer-specific mortality during the first 5-10 years after treatment that is partially offset by late effects of radiation on adjacent tissues. Since it remains uncertain if the additional volume of normal tissue that is irradiated (in order to encompass the entire breast for presumed occult disease) provides any additional benefit in reducing breast cancer recurrence, the potential detrimental effects of this additional RT would be eliminated.
Correlative Science Program
The aim of the pathology and correlative science for this trial is to identify potential predictors of selective advantage for WBI versus PBI. There will be two important aspects for correlative science studies: (1) predictors of multicentricity and local recurrence after lumpectomy without radiotherapy will have significant bearing on outcome of patients in the PBI arm, and (2) markers of radiosensitivity/resistance should be examined since, if the tumor cells are resistant to radiotherapy, the local recurrence rate will not be influenced by the field of radiation.
Editor’s Note:
Let’s get this thing done.
Prologue:
The adjuvant trastuzumab clinical research to
practice model
- Select publications
Breast Cancer Clinical Trials:
Neoadjuvant Therapy