
 |
||||||||

The adjuvant trastuzumab spectacle has another important component to consider in what could become a new oncology research model — the dissemination of important trial results into clinical practice. In addition to dramatically enhancing the molecular targeted research model, the adjuvant trastuzumab trial findings are also testimony to the efficiency of the current translation of trial results to patient care.
The following is a graphic history of how medical oncology practice changed almost instantaneously following the landmark 2005 ASCO “education session” that featured several presentations on this important therapeutic advancement.
PRE-ASCO 2005 PATTERNS OF CARE
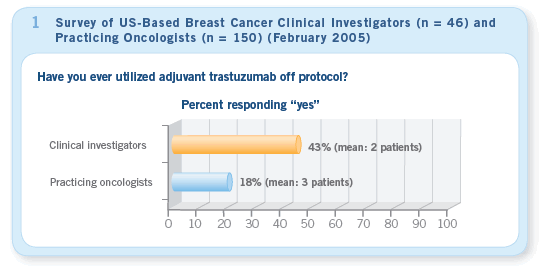
From the launch of the adjuvant trastuzumab trials in 2000 until the first release of the data in 2005, clinical investigators — with few exceptions — repeatedly emphasized that the use of trastuzumab as adjuvant therapy outside a protocol setting was not a good idea.
Frequent references were made to the stem-cell debacle as proof that the early adoption of unproven therapies is unwise and potentially dangerous.
One notable exception along the way was Dennis Slamon, who, during a 2002 interview for our Breast Cancer Update audio series, unflinchingly presented a woman with a HER2-positive, node-negative tumor who was not eligible for a clinical trial and chose to receive adjuvant TCH based on Dr Slamon’s recommendation.
However, with few exceptions, community-based oncologists seemed to heed the words of the majority of their research-focused counterparts, and prior to May 2005 both groups rarely used adjuvant trastuzumab outside of a clinical trial (Figure 1).
Of interest, our CME group conducted several anonymous polls during this time asking oncologists, “If you or a loved one were diagnosed with HER2-positive breast cancer with 10 positive nodes, would you want to receive adjuvant trastuzumab?” Many said, “Yes,” and one wonders whether we should reconsider the current mandate to practice strict evidence-based medicine when we are contemplating the use of promising therapies with modest toxicities.
PATTERNS OF CARE AFTER THE 2005 ASCO MEETING
In February 2005, when we presented cases of women with ER-negative, HER2-positive tumors and three positive nodes, none of the clinical investigators and only a small percentage of practicing oncologists would recommend trastuzumab (Figure 2a — February). Just a couple months after ASCO 2005, practice patterns had already changed dramatically. When we presented
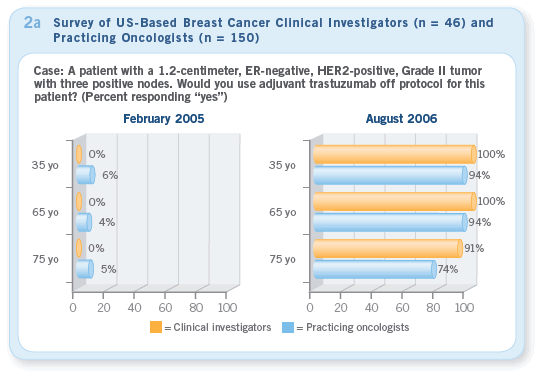
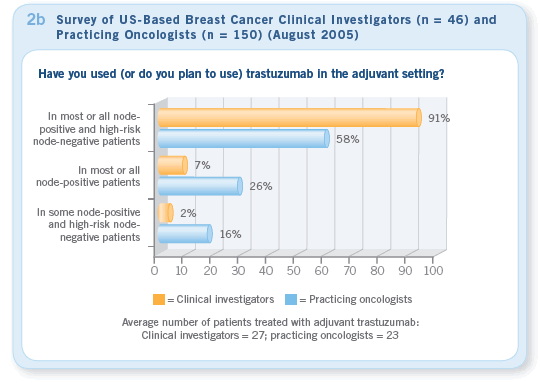
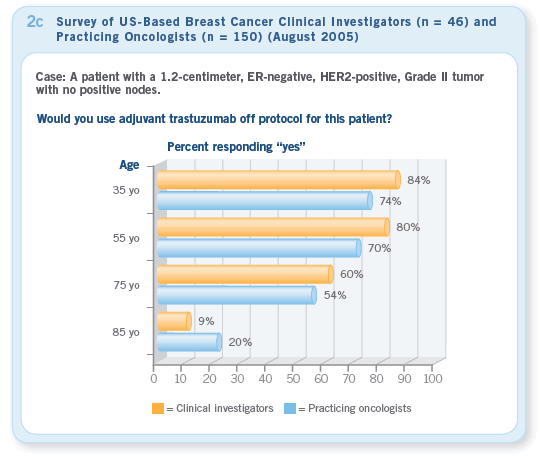
the same cases in August 2005, nearly 100 percent of clinical investigators and more than 90 percent of practicing oncologists would recommend trastuzumab for most patients (Figure 2a — August). Our August survey indicated that the majority of clinical investigators and practicing oncologists were using trastuzumab for patients with HER2-positive tumors who met the entry criteria for these trials (Figures 2b, 2c).
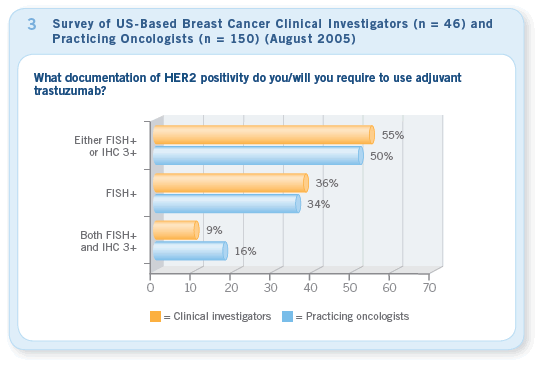
The accurate assessment of the HER2 status of a patient’s tumor is even more critical now than pre-2005 ASCO. Currently, there is a divergence of opinion as to what constitutes HER2 positivity (Figure 3), and great concerns continue to exist regarding quality control in HER2 testing (and of course ER testing). However, poor pathology quality control can lead to patients receiving inappropriate and ineffective therapy. This is a shameful travesty. The recent efforts by ASCO and the NCCN to put some pressure on the pathology community are just the beginning. This situation needs to be fixed yesterday.
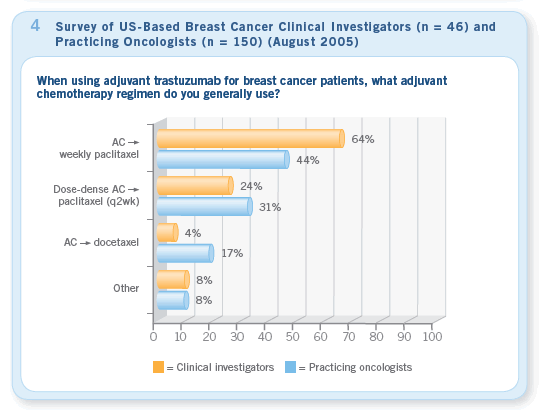
Focusing on the specific utilization of adjuvant
trastuzumab in practice, a clear trend has
emerged. Clinicians, as they so often do in
medical oncology, are most commonly following
the data and mimicking the two US-based trials
by integrating trastuzumab with a taxane after
an anthracycline regimen. It is interesting to
note that many physicians are using dose-dense
AC ![]() paclitaxel/
paclitaxel/
trastuzumab, although none of
the major reported randomized trials utilized this
chemotherapy backbone (Figure 4).
The adaptation of the dose-dense plat form for patients receiving trastuzumab is no surprise given that for the past several years, our Patterns of Care studies have demonstrated that this regimen is by far the most common adjuvant chemotherapy used for patients with node-positive disease.
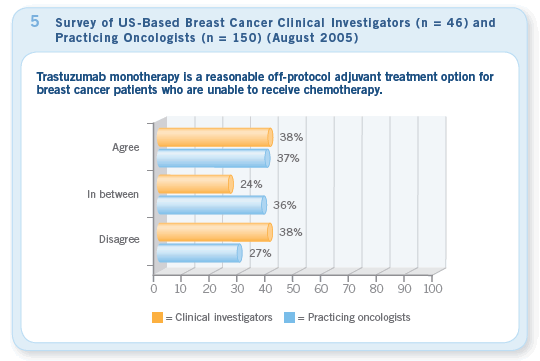
In keeping with the theme of “following the data,” virtually all clinical investigators and most practicing oncologists are prescribing adjuvant trastuzumab for the trial standard of one year and are also following the recommendations of Edith Perez and others by starting trastuzumab concurrently with taxane chemotherapy rather than using the agents sequentially. From day one, there have been many questions and much uncertainty about the use of adjuvant trastuzumab without chemotherapy, either alone (Figure 5) or with endocrine therapy for patients with ER-positive tumors.
Although this treatment approach may be appealing in some unusual situations (eg, octogenarians in suboptimal general health), the 50 percent relative reduction in relapse rate with trastuzumab/chemotherapy has most docs again trying to stick with the data and considering perhaps even a single-agent taxane along with the anti-HER2 agent for frail and elderly patients.
MAY 2005 AS A MODEL OF RESEARCH TO ONCOLOGIC PRACTICE
It was mighty interesting to have been in the middle of this seismic shift in oncology practice. As with all ASCO meetings, I had arranged to conduct interviews in Orlando with a number of breast cancer clinical investigators at the conference.
As the principal investigator of NSABP-B-31, Ed Romond from the University of Kentucky was at the top of our list to interview. I had the highly interesting opportunity to sit down with Ed immediately after the education session in which he presented the initial combined NSABP/ NCCTG data.
As one might expect, Dr Romond was ebullient and filled with energy and optimism. However, he began our conversation not by plowing through the data but by telling me what was going through his mind as he stood before the audience in Orlando poised to unleash the research equivalent of a Category 5 data storm.
Ed’s thoughts were with one of the patients he evaluated for participation in NSABP-B-31 several years ago. This young, single mother of an 11-year-old son had a HER2-positive tumor and 25 positive lymph nodes. She desperately wanted to enter the study but lived two hours from Lexington and had no means of transportation to take her to and from the frequent clinic visits the trial required.
Ed told me with considerable emotion that to solve this problem, the patient’s father — at great sacrifice — purchased a car so that she could participate in the study. This woman was randomly assigned to the trastuzumab arm and currently remains free of cancer three years later.
I imagine that many of the investigators and practicing oncologists who enrolled patients on these groundbreaking trials have similar stories to tell, and it is no wonder that a sense of intense, almost overwhelming emotion consumed the meeting hall that day in Orlando, as the enormous human implications of these data became obvious to us all.
The morning after the session, I scooped up George Sledge in the lobby of the Omni hotel at the ungodly surgical hour of 6:00 AM. It was the only time George had in his schedule for an interview, and I was deeply grateful that he met with me.
His words and Ed’s would soon be heard by thousands of oncologists and undoubtedly guided a great deal of clinical practice that summer. Those interviews, and other CME outlets and meetings, helped get the word out quickly, and surely played a role in changing clinical practice.
In my mind, this is a triumph of the interface between the clinical trials system and current methods for the communication of oncology information and perspectives.
It’s comforting to consider the life cycle that started with a series of well-designed and well executed clinical trials and ended with people like Ed’s patient perhaps avoiding relapse and death as a result. We need to repeat this cycle until we get this thing done.
Somewhere in the hills of Kentucky, this woman, with her son next to her, glides along in a loving vehicle that brought them to a place none of us could have imagined a few years ago.
Editor’s Note:
Let’s get this thing done.
Prologue:
The adjuvant trastuzumab clinical research to
practice model
- Select publications
Breast Cancer Clinical Trials:
Neoadjuvant Therapy