
 |
||||||||

COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() We’re significantly more likely to be successful
performing breast-conserving surgery after neoadjuvant
endocrine therapy than chemotherapy.
We’re significantly more likely to be successful
performing breast-conserving surgery after neoadjuvant
endocrine therapy than chemotherapy.
One reason for this is that approximately 20 to 30 percent of patients who respond well to neoadjuvant chemotherapy are left with multiple islands of tumor scattered throughout an area of the breast that corresponds to the size of the original tumor, whereas the pattern following neoadjuvant endocrine therapy is that the tumor shrinks and implodes.
The number of patients receiving neoadjuvant endocrine therapy has increased significantly, and many oncologists who have tried this approach and found that it worked have adopted this strategy.
I believe more physicians should be using this because it’s effective at downstaging some large tumors, making inoperable tumors operable. When we’re selective and treat only patients with ER-rich tumors, meaning Allred scores 6, 7 and 8, the number of patients who progress or actually fail to respond is small.
We have also learned that we can treat patients longer than three or four months with neoadjuvant therapy and see continued response.
We’ve treated patients for up to a year and found that the number of patients with a complete response continues to rise the longer we treat them. If the tumor is shrinking but still is not small enough for breast-conserving surgery at three or four months, continuing therapy will give added benefit, and eventually, most of these tumors will become small enough for breast conservation.
— J Michael Dixon, MD
![]() I believe it was a mistake to evaluate chemotherapy
rather than endocrine therapy in some of
the earlier neoadjuvant studies.
I believe it was a mistake to evaluate chemotherapy
rather than endocrine therapy in some of
the earlier neoadjuvant studies.
The perioperative phase is critical and although no evidence indicates that preoperative chemotherapy improves survival, that’s nonspecific treatment, and it doesn’t mean that neoadjuvant endocrine therapies will fail.
I view neoadjuvant endocrine treatment as a biological response modifier, and I believe using the aromatase inhibitors up front might have a greater impact on long-term outcome.
— Michael Baum, MD, ChM
SUPPORTING PROTOCOL INFORMATION
Background Information
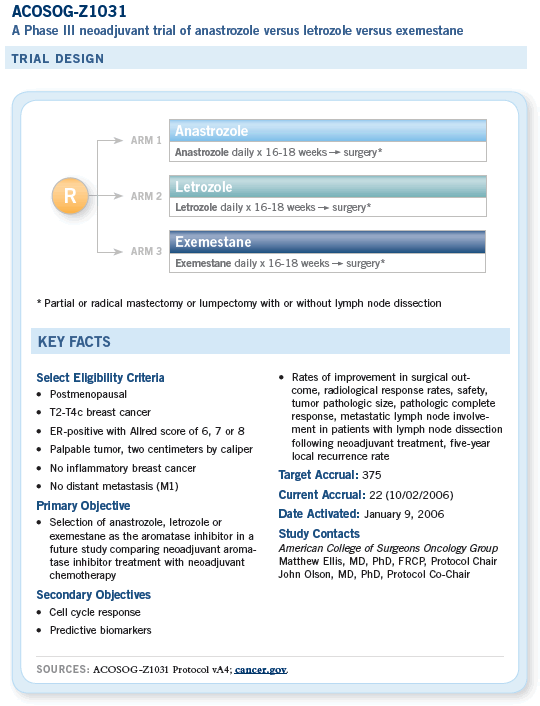
The long-term aim of the ACOSOG neoadjuvant endocrine therapy research program is to conduct a practice-setting randomized trial of neoadjuvant chemotherapy versus neoadjuvant endocrine therapy to establish neoadjuvant endocrine therapy as a routine treatment option. The objective of Z1031 is to resolve questions regarding protocol design.
The first question concerns the choice of aromatase inhibitor. The primary objective of this initial study is to compare the activity of anastrozole, letrozole and exemestane as neoadjuvant treatment.
A selection design (rather than a superiority design) has been adopted because the aim is simply to determine whether a particular aromatase inhibitor should be chosen for the comparison with chemotherapy.
If no major differences are found, an open-label choice of any of the three third-generation aromatase inhibitors may be adopted.
A second objective is to define a patient group, based on predictive biomarker research, in which the aromatase inhibitor response rate is sufficiently high to be potentially more effective than chemotherapy.
This would allow the direct comparison with chemotherapy to be based on a superiority design in favor of aromatase inhibitor treatment.
A third objective is to delineate the clinical and biomarker endpoints that will be used to rigorously judge the effectiveness of neoadjuvant endocrine therapy.
The use of neoadjuvant endocrine therapy in place of neoadjuvant chemotherapy can be justified on the basis that the patients in question (postmenopausal women with estrogen receptor-positive tumors) are receiving a form of systemic treatment that, in the adjuvant setting, is at least twice as effective as chemotherapy in providing long-term protection from relapse and death from breast cancer.
Correlative Science Program
The central theme of the primary tumor-based correlative science study is to develop an aromatase inhibitor response signature that can be translated into a widely applicable test that can be used to identify patients with a high chance of responding to aromatase inhibitor therapy in either the adjuvant or neoadjuvant setting. Since the correlative science analysis will be conducted across the three arms of the trial, the response signature that is developed will be broadly applicable and not agent specific.
COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() The novelty of NSABP-B-40 is that we’re
using pCR as an endpoint with an emphasis on
developing a molecular taxonomy to determine
whether or not we can characterize patients who
obtain a pCR as a surrogate marker to measure
outcome. Disease-free and overall survival are
not primary endpoints for NSABP-B-40. We view
it as a new mechanism to test promising agents
in the neoadjuvant setting, and we think it is an
appropriate direction to pursue, particularly with
the number of agents that are available and the
limited resources, both from a support standpoint
and a population standpoint.
The novelty of NSABP-B-40 is that we’re
using pCR as an endpoint with an emphasis on
developing a molecular taxonomy to determine
whether or not we can characterize patients who
obtain a pCR as a surrogate marker to measure
outcome. Disease-free and overall survival are
not primary endpoints for NSABP-B-40. We view
it as a new mechanism to test promising agents
in the neoadjuvant setting, and we think it is an
appropriate direction to pursue, particularly with
the number of agents that are available and the
limited resources, both from a support standpoint
and a population standpoint.
— Norman Wolmark, MD
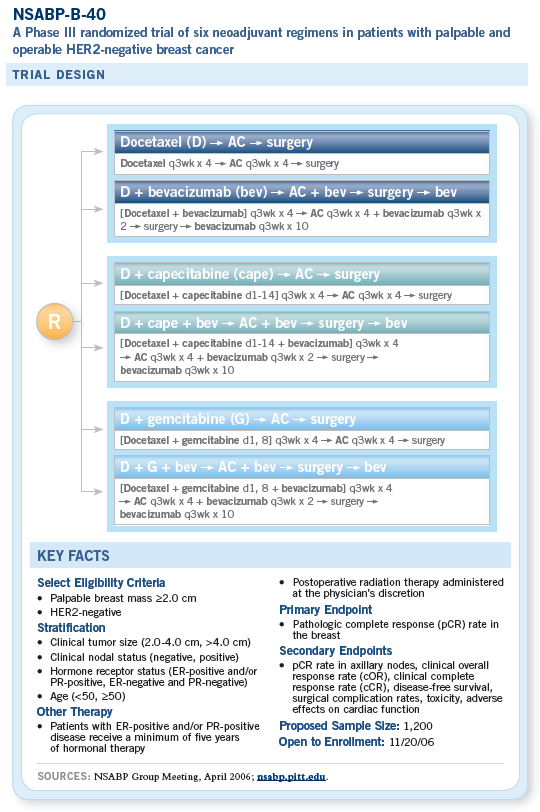
![]() NSABP-B-40 was originally going to be
a three-arm study evaluating sequential AC
followed by either docetaxel alone, docetaxel
with capecitabine or docetaxel with gemcitabine.
We were about to open the trial but decided to
modify it to incorporate bevacizumab. With that,
we reconfigured the study to move the taxane
ahead of the AC, which is the reversal of the
usual order.
NSABP-B-40 was originally going to be
a three-arm study evaluating sequential AC
followed by either docetaxel alone, docetaxel
with capecitabine or docetaxel with gemcitabine.
We were about to open the trial but decided to
modify it to incorporate bevacizumab. With that,
we reconfigured the study to move the taxane
ahead of the AC, which is the reversal of the
usual order.
Our thinking was twofold. One, the data for bevacizumab in breast cancer were with a taxane. Hence, we wanted to administer the two together as much as possible. Then, there was also concern about the possibility of increased cardiac toxicity for the anthracyclines with bevacizumab. More and more, it’s looking as if that probably isn’t going to be a concern.
— Charles E Geyer Jr, MD
![]() The NSABP-B-40 trial has evolved over a very
long period of time since we completed accrual
to B-27. From the beginning, we wanted to
evaluate the impact of adding a biologic response
modifier. We studied a lot of them over the period
of years that this has been developed, and each
one we evaluated did not pan out to have the
activity that we thought indicated that it would
be useful in this setting.
The NSABP-B-40 trial has evolved over a very
long period of time since we completed accrual
to B-27. From the beginning, we wanted to
evaluate the impact of adding a biologic response
modifier. We studied a lot of them over the period
of years that this has been developed, and each
one we evaluated did not pan out to have the
activity that we thought indicated that it would
be useful in this setting.
Certainly, bevacizumab has come along recently as a promising drug not only in the metastatic setting but also in the locally advanced neoadjuvant setting, in a study by Sandra Swain’s group at the NCI.
We are now poised to do a number of things with B-40. One is to examine different docetaxel doublets combined with capecitabine or gemcitabine as potential ways to increase response and improve patient outcomes and then to add bevacizumab to the chemotherapy, which may not only be beneficial but potentially synergistic with the chemotherapy.
We will be administering the docetaxel or docetaxel doublets first, which is different from our previous design. This is mainly to take advantage of the documented synergy between a taxane and bevacizumab and to allow us to stop the bevacizumab before surgery so we don’t run into surgical complications as a result of angiogenesis inhibition. It allows a washout period.
One of the most important outcomes of the study involves correlative science. We will be requiring the submission of four cores of tissue prior to the randomization. Two cores will be put in RNAlater® to preserve the RNA, so we can do gene-expression profiling. Another core will be put in a fixative for later use for other molecular analyses like Genomic Health’s assay, and a fourth core will be put in a medium for assessment by a chemosensitivity assay.
One of the exciting things about this study is the attempt to further understand the mechanism of action of bevacizumab. We have evidence that macroscopic tumor shrinkage may be synergistic between bevacizumab and chemotherapy, and the NCI showed nicely that cancer cells express VEGF receptor and the phosphorylation of that receptor is dramatically downregulated in patients who respond to bevacizumab, so there may be a significant effect on the tumor cells directly and on the tumor’s blood supply.
— Harry D Bear, MD, PhD
SUPPORTING PROTOCOL INFORMATION
Correlative Science Program
Pathology specimens will be collected and used to identify gene expression profiles that can predict pCR and to test a chemosensitivity assay as a predictor of pCR. Submission of core needle biopsy specimens is a pre-entry requirement for participation in B-40. Tumor blocks from any gross residual disease at the time of surgery are also required for all patients in B-40. If no gross residual disease is found at surgery, the submission is required of two unstained slides from each of the blocks from the area of the breast where the tumor had been.
Editor’s Note:
Let’s get this thing done.
Prologue:
The adjuvant trastuzumab clinical research to
practice model
- Select publications
Breast Cancer Clinical Trials:
Neoadjuvant Therapy