
 |
||||||||


COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() Adjuvant bisphosphonates are of great interest
to us because of a few intriguing European trials
that showed a probable survival benefit to adding
oral clodronate at bone metastasis doses to
treatment for early-stage breast cancer. In two
of three European trials, a survival benefit is
still apparent at 10 years. Clodronate was never
approved in the United States for any indication,
neither for treatment nor prevention of bone
metastasis, so the NSABP-B-34 study, with 3,000 patients, was designed to examine the
adjuvant question.
Adjuvant bisphosphonates are of great interest
to us because of a few intriguing European trials
that showed a probable survival benefit to adding
oral clodronate at bone metastasis doses to
treatment for early-stage breast cancer. In two
of three European trials, a survival benefit is
still apparent at 10 years. Clodronate was never
approved in the United States for any indication,
neither for treatment nor prevention of bone
metastasis, so the NSABP-B-34 study, with 3,000 patients, was designed to examine the
adjuvant question.
We hope to have those results in the next couple of years. The 10-year update of the European data was reported at the time we were designing SWOG-S0307 as the successor to the B-34 study, so we chose clodronate as the control arm, which is unconventional because it is not our standard of care in the United States. Ibandronate is another oral bisphosphonate chosen for the study, at a dose of 50 mg daily, and the third study arm is IV zoledronic acid administered monthly for the first six months and then quarterly.
Eligibility requires a high enough risk of recurrence to require treatment with endocrine therapy, chemotherapy or both. We are allowing coenrollment on virtually any other trial as long as that trial doesn’t specifically preclude it. Registration may start within 12 weeks of final surgery or chemotherapy.
For corollary studies, we are asking patients at the beginning and end of the study about their preference for receiving medication intravenously versus orally. It will be interesting to see how the different arms respond. We are also tracking patients closely to make sure that osteonecrosis of the jaw is not happening.
We’re also carefully monitoring renal function across all of the groups to see how renal function might be affected, and we are collecting blood and a tumor block from the time of the initial diagnosis in an attempt to look for things that would predict for a pattern of bone recurrence versus other sources of recurrence.
— Julie R Gralow, MD
SUPPORTING PROTOCOL INFORMATION
Background Information
Three randomized clinical trials of the oral bisphosphonate clodronate as adjuvant therapy in breast cancer have been reported, yielding conflicting results with respect to development of bone metastases and survival…
The German trial continues to show a survival benefit for the clodronate arm (80% vs 60%), with overall survival at 103 months, p = 0.04. The final analysis of the larger UK-led trial shows a statistically significant survival benefit for the clodronate arm, with a hazard ratio for survival of 0.768 (p = 0.048) that persists at 10 years of follow-up. The 10 year follow-up of the Finnish study found no significant survival difference between the groups. The intriguing but contradictory results of these three adjuvant bisphosphonate studies highlight the need for further investigation to determine whether bisphosphonates can influence the development of bone metastases and improve survival in early stage breast cancer.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) has recently completed accrual on a multicenter confirmatory trial. NSABP-B- 34 is evaluating oral clodronate for three years versus placebo in addition to standard treatment in 3,200 patients with Stage I or II breast cancer.
The North American Intergroup trial S0307 will compare newer, more potent bisphosphonate agents to clodronate. It is noteworthy that this trial design will still be valid even if the B-34 study results are not able to show a benefit for clodronate. As long as the clodronate arm is not inferior to placebo in B-34 (which is fairly unlikely provided the weight of the available evidence), the clodronate arm of the proposed trial would serve as a “reference control” against which one could still study the two newer, more potent bisphosphonates.
Clodronate is approved in Canada, Europe and Asia at the 1,600 mg per day dose for treatment of bone metastases and has been widely used for greater than a decade. It is on fast-track approval with the FDA in the US....Ibandronate has recently been approved in Europe, Central America and Asia for the treatment of bone metastases in both intravenous form (6 mg dose monthly) and oral form (50 mg daily)...Zoledronic acid is approved in the United States at the dose to be used in S0307 for the treatment of bone metastasis in multiple myeloma and all solid tumors.
Some patients may prefer oral formulations to IV formulations for reasons of comfort and convenience. Zoledronic acid is available only as an intravenous infusion, whereas ibandronate may be given either intravenously or as an oral tablet. While oral bioavailability of bisphosphonates is overall very low (≤0.5-4%), it has been shown that oral dosing of ibandronate resulting in comparable drug exposure to effective intravenous doses can be achieved and that this dose is tolerable and efficacious in inhibiting skeletal-related events.
COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() The PACCT trial is pointing us in the direction
of therapeutic individualization. We’ve known for
a long time that some of the relatively low-risk
group of patients who are estrogen receptor-positive
and lymph node-negative will benefit
from the addition of adjuvant chemotherapy to
adjuvant hormonal therapy. Based on studies
from the 1980s and 1990s, we know that
four or five women out of 100 will be alive
and disease-free a decade out if they receive
adjuvant chemotherapy in this setting. What
that automatically tells you is that we’re vastly
overtreating patients and the great majority of
patients derive no benefit from the addition of
chemotherapy to hormonal therapy. In the past,
we’ve always needed to treat an entire population
to benefit a few.
The PACCT trial is pointing us in the direction
of therapeutic individualization. We’ve known for
a long time that some of the relatively low-risk
group of patients who are estrogen receptor-positive
and lymph node-negative will benefit
from the addition of adjuvant chemotherapy to
adjuvant hormonal therapy. Based on studies
from the 1980s and 1990s, we know that
four or five women out of 100 will be alive
and disease-free a decade out if they receive
adjuvant chemotherapy in this setting. What
that automatically tells you is that we’re vastly
overtreating patients and the great majority of
patients derive no benefit from the addition of
chemotherapy to hormonal therapy. In the past,
we’ve always needed to treat an entire population
to benefit a few.
If the trial is successful, this will be the first time in a prospective, large, clinical, randomized trial that we have used modern genomic technology to help us determine therapies for patients. This will get us much closer to our goal of therapeutic individualization for patients. Our hope is to avoid unnecessary toxicity for patients who are going to do well without chemotherapy and, at the same time, pick out those patients who will receive clear survival benefit from the addition of adjuvant chemotherapy. That’s a very exciting change.
— George W Sledge Jr, MD
![]() Using archival tissue blocks from past trials,
Genomic Health and Dr Soon Paik analyzed
about 200 genes that were reported to possibly
relate to outcome in breast cancer. They narrowed
that set down to just 16 genes that could be
sorted into logical groups based on the estrogen
receptor, the HER2 protein and proliferation and
invasion characteristics of the cells.
Using archival tissue blocks from past trials,
Genomic Health and Dr Soon Paik analyzed
about 200 genes that were reported to possibly
relate to outcome in breast cancer. They narrowed
that set down to just 16 genes that could be
sorted into logical groups based on the estrogen
receptor, the HER2 protein and proliferation and
invasion characteristics of the cells.
That set of 16 genes plus five reference genes were used to see if breast cancer patients could be sorted into prognostic and predictive groups. When I say “prognostic” I mean to predict the likelihood of recurrence, and when I say “predictive” I mean to predict patients who would benefit from chemotherapy.
So these investigators examined the archival subsets and were able to determine that those 16 genes and five reference genes could be used to sort patients along a continuum they called the recurrence score, which varies from zero to 100. Using simple mathematic regression procedures, that recurrence score could then be translated into a probability of recurrence over 10 years.
The investigators were able to determine that patients who had low recurrence scores — that is, scores of 18 or lower — benefited from hormonal therapy but derived no additional benefit from the addition of chemotherapy to their hormonal therapy regimens. Conversely, patients with high recurrence scores — scores of 31 or higher — showed a clear, statistically significant and large benefit when cytotoxic chemotherapy was added to hormonal therapy — that is, tamoxifen. In the intermediate group, the group with scores between 18 and 30, no benefit was apparent from the addition of chemotherapy, but the confidence intervals — the statistical certainty of no benefit — were not established.
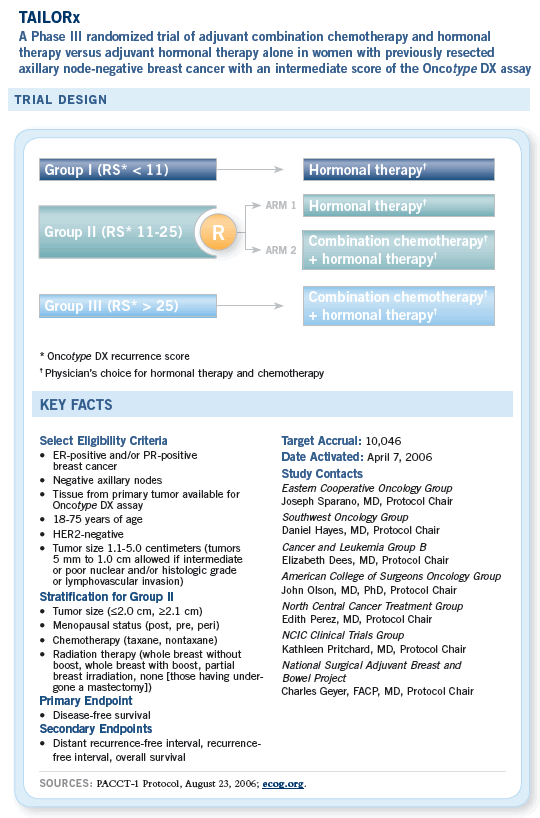
What came out of that work was the Oncotype DX assay from Genomic Health. It is commercially available and essentially allows selection of patients for hormonal therapy alone or hormonal therapy with chemotherapy in the high-risk group. In the intermediate-risk group, we’re left with some uncertainty. An Intergroup clinical trial, known as the TAILORx study, is for patients with ER-positive, node-negative, early-stage — Stage I, small Stage II — breast cancer. Patients will be randomly assigned to chemotherapy or no chemotherapy, in addition to their hormonal therapy, if they fall into that intermediate-risk group.
— Victor G Vogel, MD
![]() NSABP-B-20 included women with node-negative,
ER-positive disease. It had a three-arm
design, and patients were randomly assigned to
tamoxifen alone or tamoxifen concurrent with
either CMF or methotrexate followed by 5-FU.
Our study was a retrospective analysis of that
completed trial. We only had tissue blocks available
for approximately 30 percent of the entire
study cohort, so it’s a subset, but the subset and
the entire cohort were comparable. We repeated
the Oncotype DX assay on the tamoxifen arm
to ensure the assay was reproducible, and we
demonstrated that it is reproducible, which is
encouraging.
NSABP-B-20 included women with node-negative,
ER-positive disease. It had a three-arm
design, and patients were randomly assigned to
tamoxifen alone or tamoxifen concurrent with
either CMF or methotrexate followed by 5-FU.
Our study was a retrospective analysis of that
completed trial. We only had tissue blocks available
for approximately 30 percent of the entire
study cohort, so it’s a subset, but the subset and
the entire cohort were comparable. We repeated
the Oncotype DX assay on the tamoxifen arm
to ensure the assay was reproducible, and we
demonstrated that it is reproducible, which is
encouraging.
Importantly, we evaluated the chemotherapy arms to address whether the Oncotype DX assay recurrence score predicted chemotherapy responsiveness. We went into that study with an a priori hypothesis, based on the data presented at ASCO 2004 by Dr Luca Gianni’s group from Milan evaluating samples from a neoadjuvant trial they performed with paclitaxel and doxorubicin.
They demonstrated a correlation between the Genomic Health recurrence score and pCR rate. The higher recurrence rate correlated strongly with the higher pCR rate. The overall pCR rate was approximately 25 percent among the patients with high-risk disease, and no pCR occurred in patients with low-risk disease. We hypothesized that the benefit from chemotherapy in NSABP-B-20 would be almost negligible in patients with low-risk disease and high in patients with high-risk disease.
The results of this study are quite striking and unlike anything I’ve ever seen. The absolute benefit from chemotherapy is zero in the low-risk group and zero in the intermediate-risk group. In the high-risk group, the absolute improvement in distant recurrence at 10 years is 28 percent, or a relative risk reduction of 75 percent. The data with the low-risk group are, in a sense, not relevant because the baseline risk after tamoxifen is so low — 6.8 percent — that it’s a moot point whether they need chemotherapy or not. In the intermediate-risk group the confidence interval overlaps with one, so whether patients with intermediate-risk disease gain any benefit or not remains a question.
These data provide an important paradigm shift in the way we think about clinical trial design and patient management. So far, in most clinical trial designs, we have presumed that the proportional benefit or incremental gain would be the same degree for patients with low-risk and high-risk disease. All statistical sample size calculations are based on that assumption, but now we have to change that. It forces us to think about the clinical trial designs by which we preselect patients who are at high risk because those are the patients who will benefit. We already knew from other studies that patients with ER-positive disease do not benefit much from chemotherapy.
In the neoadjuvant trials, the pCR rate is much lower for ER-positive tumors. This study definitely shows that, based on genes related to proliferation or estrogen receptor, we can select patients who are the best candidates for chemotherapy trials.
— Soonmyung Paik, MD
![]() In the B-20 study, patients with an intermediate
recurrence score also did not seem to derive
much benefit. The 10-year distant recurrence-free
survival was approximately 90 percent both
for patients treated with tamoxifen alone and
those treated with tamoxifen and chemotherapy.
In the B-20 study, patients with an intermediate
recurrence score also did not seem to derive
much benefit. The 10-year distant recurrence-free
survival was approximately 90 percent both
for patients treated with tamoxifen alone and
those treated with tamoxifen and chemotherapy.
However, in that group of patients, the confidence intervals around the estimates were somewhat wide, so we could not exclude some benefit. In fact, the odds ratio was about 0.6, so a reduction of up to 40 percent is possible.
What was interesting was that the benefit was seen in the patients with a high recurrence score. Among those patients, the absolute improvement in distant disease-free survival with chemotherapy was 28 percent, or a 75 percent relative reduction in the odds of recurrence. The group that received tamoxifen alone had a 60 percent distant disease-free survival rate at 10 years, and it was 88 percent for the group that received tamoxifen with chemotherapy. We’ve never seen such differences in any subset of patients with breast cancer. I like to quote what George Sledge said when he saw these data: “This makes CMF look like a targeted regimen.” That’s true. In other words, we found a signature that predicts a huge benefit from a regimen that otherwise was almost ready to become obsolete.
— Eleftherios P Mamounas, MD, MPH
![]() The reason we are conducting the TAILORx trial
is that we are in enormous equipoise about the
addition of chemotherapy for the intermediate
group. I believe we all agree that the addition of
chemotherapy for the low recurrence score group
is below our radar screen in terms of benefit,
and most of us also agree that patients with
high recurrence scores have at least a five to six
percent or higher absolute reduction in recurrence
rates. Those are the patients for whom we
would probably recommend chemotherapy.
The reason we are conducting the TAILORx trial
is that we are in enormous equipoise about the
addition of chemotherapy for the intermediate
group. I believe we all agree that the addition of
chemotherapy for the low recurrence score group
is below our radar screen in terms of benefit,
and most of us also agree that patients with
high recurrence scores have at least a five to six
percent or higher absolute reduction in recurrence
rates. Those are the patients for whom we
would probably recommend chemotherapy.
But for the intermediate group, whether we define it as a recurrence score of 11 or 18, we are in great equipoise. That is especially true because the aromatase inhibitors may be more effective than tamoxifen, so patients have a better prognosis than the patients in the NSABP study. I also believe that doxorubicin and the taxanes will be more effective in patients with lower ER and higher HER2 levels.
So depending on where you are in that intermediate group, you may have a better prognosis than we think you have, but you may have a higher proportional reduction than that achieved with CMF. The randomized portion of that trial is critical.
— Daniel F Hayes, MD
![]() The TAILORx trial is following up on the
findings of the value of the Oncotype DX assay in assessing the risk of recurrence and predicting
the benefit from chemotherapy. It’s an interesting
and ambitious trial that is scientifically
compelling and something we would like to see
completed.
The TAILORx trial is following up on the
findings of the value of the Oncotype DX assay in assessing the risk of recurrence and predicting
the benefit from chemotherapy. It’s an interesting
and ambitious trial that is scientifically
compelling and something we would like to see
completed.
— Norman Wolmark, MD
![]() The TAILORx trial is meant to ask a very
practical question borne of the data that have
been generated by Genomic Health evaluating a
limited number of genes in paraffin-embedded
tissue. The real-time quantitative PCR is used
to generate a score of the risk of recurrence for
patients with early-stage, ER-positive, node-negative
breast cancer. Based on that score, one
can make decisions about adding chemotherapy
to the standard hormone therapy that is given.
The TAILORx trial is meant to ask a very
practical question borne of the data that have
been generated by Genomic Health evaluating a
limited number of genes in paraffin-embedded
tissue. The real-time quantitative PCR is used
to generate a score of the risk of recurrence for
patients with early-stage, ER-positive, node-negative
breast cancer. Based on that score, one
can make decisions about adding chemotherapy
to the standard hormone therapy that is given.
The TAILORx trial focuses our attention in the areas where we have the greatest degree of uncertainty. We are now asking that question in an identified subset of patients where we would predict that the risk of recurrence is modest and the benefits of chemotherapy are modest. Therefore, it’s important to determine whether there is benefit.
— Clifford Hudis, MD
SUPPORTING PROTOCOL INFORMATION
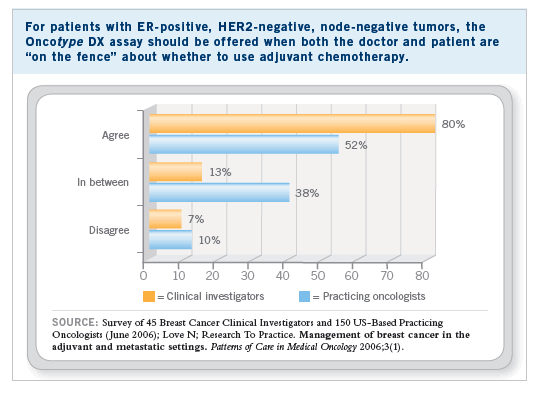
Although several distinct molecular signatures identified by differing methodologies have been developed that may serve as useful prognostic markers, we have chosen to utilize the Oncotype DX Breast Cancer Assay in this trial for the following reasons: 1) it is a standardized, multigene RT-PCR-based molecular technique performed in a single laboratory, 2) it may be applied to tissue specimens routinely processed in clinical pathology laboratories, 3) it has received CLIA approval in the United States to “...assess the likelihood that a women’s breast cancer will...recur...” (genomichealth.com), 4) it more reliably predicts prognosis than standard clinical criteria in patients with ER-positive, node-negative disease, including tumor size, histologic grade and age, 5) its performance has been validated in a large population-based study, and 6) preliminary data indicate that it predicts benefit from adjuvant chemotherapy. Patients with ER-positive, axillary node-negative breast cancer account for nearly one half of all breast cancer diagnosed in the United States, and this is the group in which more patients unnecessarily receive adjuvant chemotherapy.

COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() We’re excited about the trial, which is an equivalence
study. Some preliminary data suggest that
oral capecitabine is as good as standard therapy
in metastatic disease. It would be nice if we had
an oral regimen because I think people would
rather be at home than in our clinics all the time.
We have a quality-of-life endpoint, and we’re
collecting data from approximately the first 300
patients. We’re also going to collect tumor blocks
to see if we can predict how these older patients
do with chemotherapy.
We’re excited about the trial, which is an equivalence
study. Some preliminary data suggest that
oral capecitabine is as good as standard therapy
in metastatic disease. It would be nice if we had
an oral regimen because I think people would
rather be at home than in our clinics all the time.
We have a quality-of-life endpoint, and we’re
collecting data from approximately the first 300
patients. We’re also going to collect tumor blocks
to see if we can predict how these older patients
do with chemotherapy.
In CALGB-49907, capecitabine is given at a dose of 2,000 mg/m2 per day in divided doses for 14 consecutive days every three weeks for six cycles. We initially escalated the dose to 2,500 mg/m2, but we elected to reduce it because of severe toxicity. I believe this lower dose is certainly adequate.
Based on our experience with a dihydropyrimidine dehydrogenase-deficient patient, we amended the protocol to identify these patients. We now have women come in between days four and six of the first cycle and again several days later for “mini checks.” We do this to make sure we don’t miss patients who may have profound toxicity early. These checks will enable us to stop the drug early and avoid serious toxicity. Our assessment is that capecitabine is a reasonably safe drug, but patients need to be informed. Doctors who don’t frequently use capecitabine need to be aware of this early toxicity, and older patients should be contacted and assessed.
We are gathering excellent quality-of-life data and collecting adherence data with an electronic pill bottle. We are also evaluating some incredible laboratory science, including genes that might tell us about toxicity, such as levels of thymidine phosphorylase, thymidylate synthase and dihydropyrimidine dehydrogenase. We’ll be storing all the blocks for future work.
Although it’s a little early for me to predict how to compare these regimens, I believe patients may perceive that capecitabine is a little easier to take because it is oral and not associated with alopecia.
— Hyman B Muss, MD
![]() In addition to the more familiar ER, PR and
HER2 markers, we are evaluating some interesting
predictive and prognostic markers and
other biological markers.
In addition to the more familiar ER, PR and
HER2 markers, we are evaluating some interesting
predictive and prognostic markers and
other biological markers.
We are also examining how these drugs are metabolized in the elderly population. The data from the metastatic setting provided the rationale for selecting capecitabine for this trial — these trials compared capecitabine to single-agent paclitaxel and to CMF and demonstrated benefits from capecitabine in time to progression.
— Maria Theodoulu, MD
SUPPORTING PROTOCOL INFORMATION
Background Information
Women 70 years and older are not represented in current adjuvant trials. In the meta-analysis of 18,000 women entered into randomized trials of adjuvant chemotherapy, only 600 patients (3%) 70 years and older were entered into these trials. Although this sample size was insufficient to determine the benefits of chemotherapy in this age group, combination chemotherapy regimens significantly lowered the risk of recurrence by 20% (SD 3%) and the risk of dying of breast cancer by 11% (SD 3%) in patients 50 to 69 years.
Since it is unlikely that the proportional benefits of chemotherapy for patients age 70 years and older are different than for postmenopausal women 50 to 69 years old, there is no reason to suspect that older women would not have similar risk reductions.
A recent randomized phase II trial, comparing single agent capecitabine and CMF as first-line therapy in patients with metastatic breast cancer who were 55 years and older (median age 69 years), demonstrated that the response rate to capecitabine alone (25%) at a dose of 2,510 mg/m2 per day for 14 days every 3 weeks was superior to intravenous CMF (16%). Grade 3 or 4 hand-foot syndrome was seen in 16% of patients on capecitabine and none on CMF, grade 3 or 4 diarrhea in 8% with capecitabine and 3% with CMF, and grade 3/4 hematological toxicity in 20% with capecitabine and 47% with CMF. In another phase II randomized trial comparing capecitabine in the same dose and schedule as above with paclitaxel 175 mg/m2 every three weeks, the response rate was 36% for 22 patients on capecitabine and 21% for 22 patients on paclitaxel. These data suggest that the efficacy of capecitabine in patients with metastatic disease is similar to CMF or paclitaxel.
COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() Compared to the AC regimen that is widely
used in the adjuvant setting, paclitaxel offers
a couple of advantages. It should not cause
congestive heart failure, which is a very specific
anthracycline toxicity. Second, it may not cause
the same long-term risk of leukemia and myelodysplastic
syndromes that are seen with AC. We therefore sought to determine whether we
could substitute single-agent paclitaxel for AC in
patients at lower risk.
Compared to the AC regimen that is widely
used in the adjuvant setting, paclitaxel offers
a couple of advantages. It should not cause
congestive heart failure, which is a very specific
anthracycline toxicity. Second, it may not cause
the same long-term risk of leukemia and myelodysplastic
syndromes that are seen with AC. We therefore sought to determine whether we
could substitute single-agent paclitaxel for AC in
patients at lower risk.
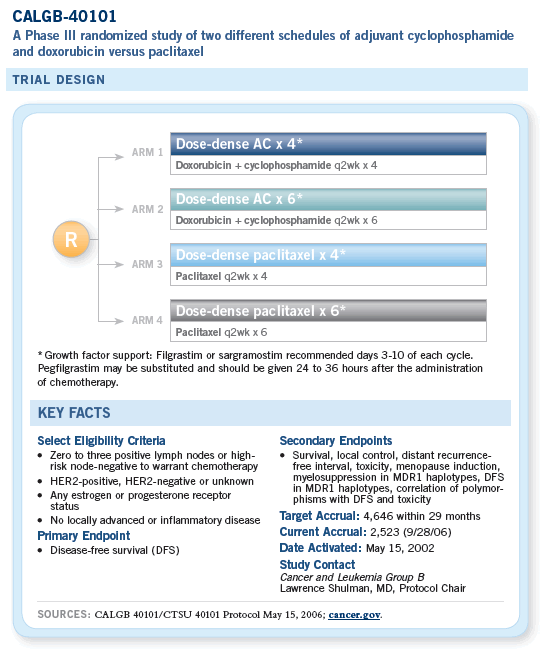
The second component of CALGB-40101 asked a more basic question, which was whether a few more cycles of chemotherapy are better than a few less. So this study not only compares paclitaxel versus AC, it also compares four cycles of therapy versus six.
After this study began, we received the results for CALGB-9741, which identified the benefits of giving chemotherapy every two weeks rather than every three weeks with the important advantages of improvements in disease-free and overall survival and the shortening of time that patients are on therapy. There was also the paradoxical but important result that the every other-week chemotherapy is a bit less toxic than the every third-week therapy. We therefore modified 40101 so that it was symmetrical and even, with every patient getting simply every two-week therapy, four or six cycles, AC or paclitaxel.
— Clifford Hudis, MD
SUPPORTING PROTOCOL INFORMATION
Background Information
Adjuvant trastuzumab will be allowed only in patients whose tumors are HER2 positive by either IHC 3+ staining or gene amplification by FISH... A fifty-two week course of trastuzumab will be permitted for all HER2-positive patients. For patients enrolled in the paclitaxel arms, trastuzumab may be administered concurrently with paclitaxel. The concurrent use of an anthracycline and trastuzumab is not acceptable.
SUPPORTING PROTOCOL INFORMATION
Background Information
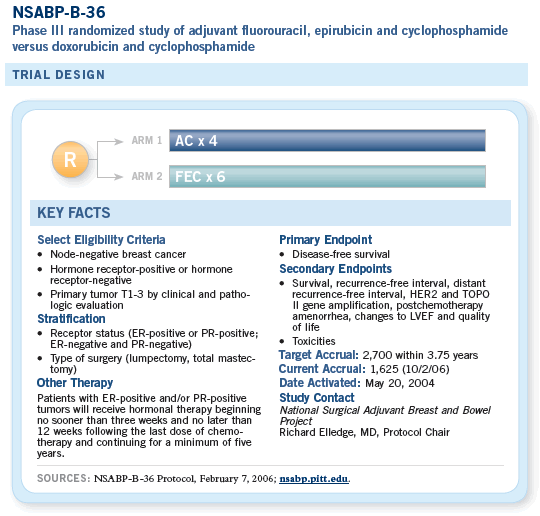
On the basis of findings from our previously published studies of NSABP trials B-11 and B- 15 as well as from other published studies, we hypothesize that clinical benefit from six cycles of FEC over four cycles of AC may be restricted to a cohort whose tumors have amplification of HER-2 and/or topoisomerase-2-alpha (Topo-II). Tissue microarrays will be generated from the submitted blocks and FISH (fluorescence in-situ hybridization) will be used to examine the presence or absence of gene amplification for HER2 and Topo-II.
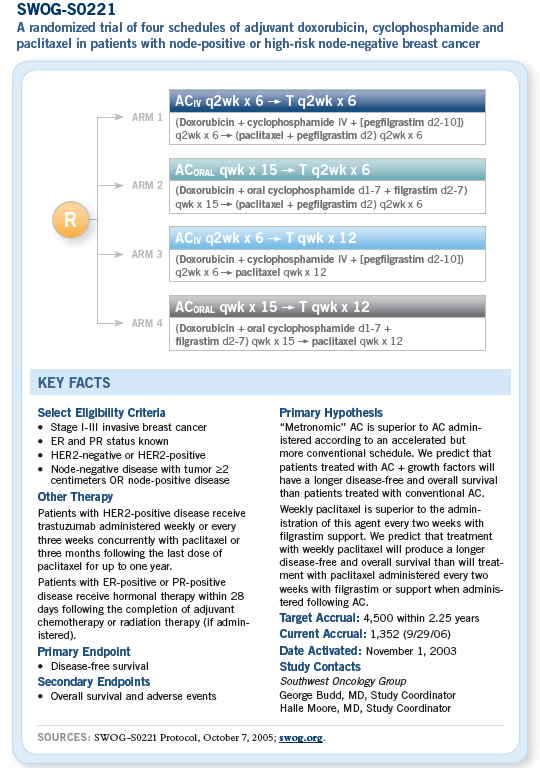
COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() In the SWOG-0221 study, AC is administered
in either a dose-dense manner with pegfilgrastim
or what might be described as a metronomic
schedule with filgrastim. Both schedules are
then followed by paclitaxel. We chose six cycles
of AC and paclitaxel in the control arms for
several reasons. By imposing similar durations of
treatment in all arms, we avoid wondering later
whether an inferior outcome in any arm reflected
the duration of treatment.
In the SWOG-0221 study, AC is administered
in either a dose-dense manner with pegfilgrastim
or what might be described as a metronomic
schedule with filgrastim. Both schedules are
then followed by paclitaxel. We chose six cycles
of AC and paclitaxel in the control arms for
several reasons. By imposing similar durations of
treatment in all arms, we avoid wondering later
whether an inferior outcome in any arm reflected
the duration of treatment.
Data suggest six cycles is superior, although this is still controversial. This more continuous schedule may provide a good chemotherapy base upon which to add other anti-angiogenic approaches. Evidence suggests that with the maximum tolerated dose schedule, a burst of vasculogenesis occurs between cycles. Hematopoietic growth factors possibly augment that, but it is unclear whether that occurs with weekly doxorubicin and daily cyclophosphamide.
— G Thomas Budd, MD
![]() The initial trial design of SWOG-S0221 was
based on two small pilot studies that demonstrated
that highly dose-dense therapy for 20 to
24 weeks — with weekly doxorubicin and daily
oral cyclophosphamide requiring G-CSF support
— produced promising results in patients with
node-positive disease. Patients with a median of
four positive nodes had an 86 percent five-year
disease-free survival, which compared favorably
to the standard NSABP AC regimen in a similar
population.
The initial trial design of SWOG-S0221 was
based on two small pilot studies that demonstrated
that highly dose-dense therapy for 20 to
24 weeks — with weekly doxorubicin and daily
oral cyclophosphamide requiring G-CSF support
— produced promising results in patients with
node-positive disease. Patients with a median of
four positive nodes had an 86 percent five-year
disease-free survival, which compared favorably
to the standard NSABP AC regimen in a similar
population.
Then the results of CALGB-9741 changed the landscape of clinical research in the adjuvant setting. Members of the Intergroup share a strong desire to build upon that trial, which showed the every two-week administration of AC and paclitaxel, with G-CSF support, was better than the every three-week schedule.
The logical next step would be a comparison of every two-week AC and our weekly doxorubicin and daily cyclophosphamide regimen — “dose dense versus dose denser.” The evaluation of weekly paclitaxel was suggested by the outcome of the MD Anderson neoadjuvant study, which randomly assigned patients to every three-week versus weekly paclitaxel, with the FAC component constant in both arms. A major advantage was seen in the pathologic complete response — 28 versus 14 percent — for patients who received weekly paclitaxel.
Growth factor support is used in each arm of the trial. Pegfilgrastim — the pegylated form of G-CSF — is utilized in the every two-week arms, and patients treated with the weekly doxorubicin and daily cyclophosphamide regimen will receive filgrastim because we do not have experience with pegfilgrastim and concurrent chemotherapy and the FDA will not allow it.
The study is a two-by-two factorial design. We will not have enough statistical power to formally test for superiority of each of the four arms, but we have more than enough power to test for the weekly versus every two-week approaches, which was the same statistical approach taken in CALGB-9741. The study will accrue approximately 4,500 patients, which is almost twice as many as CALGB-9741.
— Robert B Livingston, MD
![]() The SWOG-S0221 study builds upon CALGB-9741, but it makes several important modifications.
First, it assumes that six cycles are better
than four, so the baseline is six cycles of dose-dense
AC followed by six cycles of paclitaxel,
every two weeks.
The SWOG-S0221 study builds upon CALGB-9741, but it makes several important modifications.
First, it assumes that six cycles are better
than four, so the baseline is six cycles of dose-dense
AC followed by six cycles of paclitaxel,
every two weeks.
Using a factorial design and random assignment, half the patients will receive the metronomic schedule for AC, which is a regimen that involves weekly low-dose doxorubicin and oral daily cyclophosphamide. Some preoperative data are showing a very high response rate for patients treated with that regimen specifically, and there are reasons to hypothesize that it could be superior.
The study will also address whether low-dose weekly paclitaxel for twelve weeks might be superior to every two-week therapy the way that it was superior to every three-week therapy. The SWOG study is a very important effort that allows us to “dot the Is and cross the Ts” about whether there is an optimal way to deliver these drugs to patients.
— Clifford Hudis, MD
![]() SWOG-S0221 is an adjuvant trial for what
we’re calling patients at high risk — generally
they have node-positive disease but could also
have node-negative disease if they have a higher
than average risk. It’s a two-by-two design asking
what is the best way to administer an anthracycline-
based combination and what is the best
way to administer a taxane.
SWOG-S0221 is an adjuvant trial for what
we’re calling patients at high risk — generally
they have node-positive disease but could also
have node-negative disease if they have a higher
than average risk. It’s a two-by-two design asking
what is the best way to administer an anthracycline-
based combination and what is the best
way to administer a taxane.
It’s modeled after the CALGB dose-dense experience. So for the standard arm with both the AC and the paclitaxel, the question is an every two-week AC or an every two-week paclitaxel. The experimental arm is a metronomic dosing schedule that was piloted in Seattle and then through SWOG, in which the anthracycline is administered in a smaller weekly dose.
The cyclophosphamide is oral and is administered continuously on a daily basis. The taxane section is weekly. So AC dose dense versus AC metronomic is one question, and paclitaxel every two weeks at the 175 mg/m2 dose versus paclitaxel weekly at the 80 mg/m2 dose is another.
We’re interested in whether there is potential with this lower, metronomic, continuous dosing to have an even greater impact maybe not solely on the tumor cells but also on the angiogenic properties of the tumor environment.
Currently, we’re seeing more neutropenia with the dose-dense AC, which we’re administering with pegfilgrastim, than we are with the metronomic regimen, which we’re administering with filgrastim six out of seven days a week.
I will remind you that for a variety of complicated reasons, the AC and the taxane dose-dense schedule are administered for six rather than four cycles. So it is an expansion of the dose-dense concept with a few extra cycles.
The decision part-way through the development of the study was that they did not want the time on the study drug to be different, so they added a few more cycles of the AC and the taxane.
— Julie R Gralow, MD
![]() This Intergroup trial SWOG-S0221 compares a
regimen of oral cyclophosphamide and a weekly
anthracycline to the dose-dense every two-week
doxorubicin and cyclophosphamide (AC) regimen
used in CALGB-9741. It also compares dose-dense
every two-week to weekly paclitaxel.
This Intergroup trial SWOG-S0221 compares a
regimen of oral cyclophosphamide and a weekly
anthracycline to the dose-dense every two-week
doxorubicin and cyclophosphamide (AC) regimen
used in CALGB-9741. It also compares dose-dense
every two-week to weekly paclitaxel.
Frankly, I don’t know which regimens will be better, and I have pure equipoise on this study. The trial is not as clean a comparison as the one in CALGB-9741, in which all the doses were kept exactly the same and only the schedule was varied. For the dose-dense regimens, the additional expense associated with filgrastim and pegfilgrastim is a real and important concern.
— Larry Norton, MD
![]() SWOG-S0221 is an important study, particularly
with regard to the best way to administer
paclitaxel. Weekly paclitaxel is an interesting
regimen, and it’s logical to compare it to a dose-dense
regimen that is probably more expensive.
SWOG-S0221 is an important study, particularly
with regard to the best way to administer
paclitaxel. Weekly paclitaxel is an interesting
regimen, and it’s logical to compare it to a dose-dense
regimen that is probably more expensive.
On the other hand, weekly paclitaxel will require weekly visits to the hospital, which might not be easy. In the meantime, ECOG-1199 provides a head-to-head comparison of every three-week paclitaxel, every three-week docetaxel, weekly paclitaxel and weekly docetaxel.
— Martine J Piccart-Gebhart, MD, PhD
SUPPORTING PROTOCOL INFORMATION
Correlative Science Program
Tissue blocks will be obtained to serve as a resource for future correlative studies. Examples of such studies might include the following: tumor microvessel density or tumor VEGF expression by immunohistochemistry.
The inferior survival outcomes of women of African ancestry (AA) with breast cancer after adjustment for multiple variables mandates exploration of treatment details, molecular, biologic, pharmacogenetic and hormonal hypotheses. To lay the groundwork for future studies, we will further explore the etiology of the observed ethnic/racial differences in breast cancer outcomes. This project will address differences in estrogen synthesis and metabolism and variability in chemotherapeutic drug metabolism as factors in racial differences in survival.
There is widespread use of antioxidants and other dietary supplements among cancer patients, used with the intentions and hopes that supplement use will maintain overall health, decrease treatment- associated toxicity and increase treatment efficacy. However, there are no existing empirical data to support the notion that antioxidant supplement use can decrease toxicity associated with treatment, and it is unclear if vitamin use has any impact on treatment efficacy, either to inhibit it or to enhance it.
We will query women enrolled in this study about supplement use and evaluate it in relation to toxicity and recurrence. We will also evaluate if variants (polymorphisms) in genes that impact levels of oxidative stress will affect toxicity and disease-free survival or modify relationships between supplement use and treatment outcomes.
Continue
Page 1 of 2
Editor’s Note:
Let’s get this thing done.
Prologue:
The adjuvant trastuzumab clinical research to
practice model
- Select publications
Breast Cancer Clinical Trials:
Neoadjuvant Therapy