
 |
||||||||
COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() NSABP-B-38 is a very practical study. At the
time we designed this trial, we talked a lot about
also studying bevacizumab. Even before the data
for ECOG-E2100 came out, we were excited
about bevacizumab and thought that would be
the biologic agent that would be important for the adjuvant setting. However, the drug was not
felt to be ready for the adjuvant setting. So we
decided to ask a practical question.
NSABP-B-38 is a very practical study. At the
time we designed this trial, we talked a lot about
also studying bevacizumab. Even before the data
for ECOG-E2100 came out, we were excited
about bevacizumab and thought that would be
the biologic agent that would be important for the adjuvant setting. However, the drug was not
felt to be ready for the adjuvant setting. So we
decided to ask a practical question.
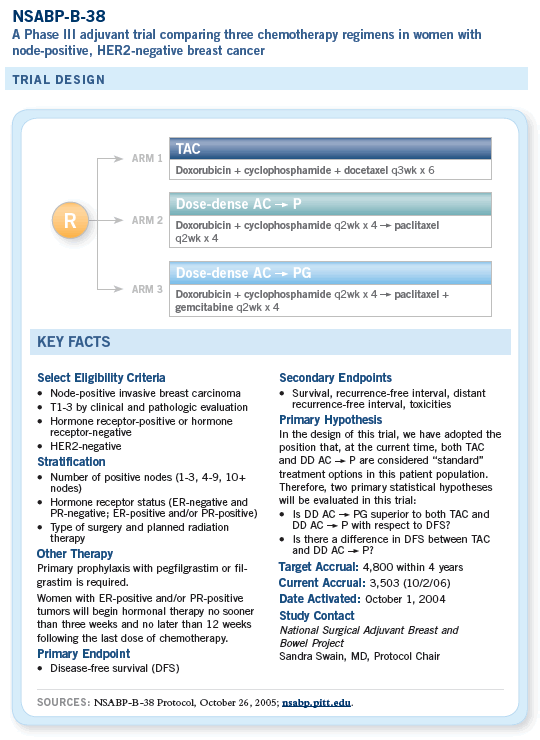
The dose-dense data had been presented and showed a one or two percent survival benefit, and that seemed to be a popular regimen, with more than half the country using it. We also considered the BCIRG 001 data evaluating TAC versus FAC, which showed a very positive result with much longer follow-up. At that time, I felt that the docetaxel was a more effective taxane, not having the ECOG-E1199 data yet.
So we decided to compare TAC to dose-dense chemotherapy. Then Kathy Albain presented gemcitabine/paclitaxel data, which showed a small survival benefit when you add gemcitabine.
We decided to have another arm so we could improve outcomes, if possible, if the dose-dense regimen turned out to be best or equivalent to TAC.
When the adjuvant trastuzumab data came out, we decided to exclude patients who were HER2-positive. We didn’t have that many patients on the trial who were HER2-positive to begin with, because the NSABP-B-31 study was running concurrently and most of the patients would have gone on that study.
— Sandra M Swain, MD
![]() BCIRG 001, evaluating TAC versus FAC, and
the CALGB-9741 dose-dense trial of AC/paclitaxel
are two key adjuvant trials. Currently, our
view is that TAC appears to be the optimal way to
administer an anthracycline/docetaxel regimen,
and dose-dense AC/paclitaxel is the optimal way
to administer those agents.
BCIRG 001, evaluating TAC versus FAC, and
the CALGB-9741 dose-dense trial of AC/paclitaxel
are two key adjuvant trials. Currently, our
view is that TAC appears to be the optimal way to
administer an anthracycline/docetaxel regimen,
and dose-dense AC/paclitaxel is the optimal way
to administer those agents.
Which is better? It’s impossible to answer that question without performing a clinical trial, which is why we developed NSABP-B-38.
It’s a pragmatic design in which we regard TAC as our control arm. A clear advantage of dose-dense therapy is that it is so well tolerated, and it affords the opportunity to add a fourth drug to the paclitaxel. TAC is a maximally tolerated regimen. You really can’t push it much more, so we sought a candidate drug to combine with paclitaxel.
— Charles E Geyer Jr, MD
![]() NSABP-B-38 was motivated by the debate
and controversy surrounding the optimal chemotherapy
regimen for treating node-positive
disease. The concept of dose density, which
came to the forefront in 2002, has made remarkable
headway from a popularity standpoint. The
real question in our minds was whether we can
incorporate it and compare it to what others
consider to be a standard of care, which is the
TAC regimen.
NSABP-B-38 was motivated by the debate
and controversy surrounding the optimal chemotherapy
regimen for treating node-positive
disease. The concept of dose density, which
came to the forefront in 2002, has made remarkable
headway from a popularity standpoint. The
real question in our minds was whether we can
incorporate it and compare it to what others
consider to be a standard of care, which is the
TAC regimen.
So we have a three-arm trial in which, in essence, the control arm is six cycles of TAC compared to “Nortonian” dose-dense chemotherapy and the third arm attempts to improve on the regimen by adding a gemcitabine doublet, also given in a dose-dense regimen.
This trial has been popular. It started in October 2004, and the required sample size is approximately 4,800. To date, we have more than 3,300 patients accrued.
— Norman Wolmark, MD
![]() A paper in Seminars in Oncology in the mid-1980s indicated that the primary problem in
Gompertzian growth is not cell kill but rather
regrowth between cycles.
A paper in Seminars in Oncology in the mid-1980s indicated that the primary problem in
Gompertzian growth is not cell kill but rather
regrowth between cycles.
While therapy gets us closer to the cure limits, you have to get below a small number of cells to prevent regrowth, and regrowth occurs faster as you move away from that limit.
A rebound effect is evident, and the key is to inhibit that regrowth. One of the simplest ways to address regrowth is to move the doses of therapy close enough together to have less regrowth between cycles.
This is extremely powerful in Gompertzian kinetics, as long as you can drive the tumor toward that cure limit. In the adjuvant setting, when you’re probably close to the cure limit, you can achieve dramatic benefits by giving the doses closer together in time.
— Larry Norton, MD
![]() On the basis of the available data, one can
consider TAC to be a standard of care, as is the
dose-dense regimen of doxorubicin and cyclophosphamide
followed by paclitaxel, for patients
with resected node-positive breast cancer.
However, the exclusion of patients older than 70
years and the toxic effects associated with TAC
in the BCIRG trial cannot be minimized. With
this regimen, prophylactic growth-factor support
is necessary to ameliorate myelosuppression and
febrile neutropenia.
On the basis of the available data, one can
consider TAC to be a standard of care, as is the
dose-dense regimen of doxorubicin and cyclophosphamide
followed by paclitaxel, for patients
with resected node-positive breast cancer.
However, the exclusion of patients older than 70
years and the toxic effects associated with TAC
in the BCIRG trial cannot be minimized. With
this regimen, prophylactic growth-factor support
is necessary to ameliorate myelosuppression and
febrile neutropenia.
A recommendation for the selection of one regimen over the other must await completion of the prospective National Surgical Adjuvant Breast and Bowel Project trial B-38, for which the accrual of data is expected to be complete in the next few years.
— Edith A Perez, MD.
N Engl J Med 2005;352(22):2346-8.
![]() I believe that TAC without growth factors is
more toxic than dose-dense AC. We have data
from a trial in Spain in which Miguel Martin
treated node-negative disease with TAC or FAC.
Early in the trial they thought, “For node-negative
disease, TAC is quite tough,” and they mandated
G-CSF.
I believe that TAC without growth factors is
more toxic than dose-dense AC. We have data
from a trial in Spain in which Miguel Martin
treated node-negative disease with TAC or FAC.
Early in the trial they thought, “For node-negative
disease, TAC is quite tough,” and they mandated
G-CSF.
At that point, they found that the tolerability increased dramatically. It’s not a randomized trial, and it’s an intervention halfway through, but they found that not only did the febrile neutropenia rate drop, but the mucositis, fatigue and diarrhea decreased as well.
In addition, the quality-of-life decrements that come with chemotherapy were less after G-CSF was initiated.
I agree that “naked” TAC without growth factors is probably tougher than dose-dense therapy with growth factors. However, I believe that difference would be much less if you used primary prophylaxis with pegfilgrastim or filgrastim. I would suggest that if you are going to use it, use it with growth factor support.
— John Mackey, MD
![]() When people say that the addition of dose-dense
scheduling in CALGB-9741 doesn’t yield
much among patients with ER-positive disease,
they’re not comparing apples to apples when they
then assess the TAC-FAC data.
When people say that the addition of dose-dense
scheduling in CALGB-9741 doesn’t yield
much among patients with ER-positive disease,
they’re not comparing apples to apples when they
then assess the TAC-FAC data.
The TAC-FAC trial demonstrated hazard rates for risk reductions, which looked about the same in the ER-positives and the ER-negatives. The FAC control arm, of course, includes no paclitaxel or docetaxel.
You can’t say that each individual step is or is not significant vis-à-vis another separate randomized trial. You can’t compare these regimens head to head. If you were to argue that you know to use TAC instead of dose-dense AC paclitaxel for a patient with ER-positive, node-positive disease, then you’re presuming to know the results of NSABP-B-38.
I would argue that there is equipoise on this question and that either regimen is entirely appropriate for patients with ER-positive disease.
— Clifford Hudis, MD
SUPPORTING PROTOCOL INFORMATION
Background Information
In a sense, TAC represents the current
optimal program of a taxane, doxorubicin,
and cyclophosphamide combination. DD
AC ![]() P represents the current optimal program
of a sequential taxane following a doxorubicin
and cyclophosphamide program...
P represents the current optimal program
of a sequential taxane following a doxorubicin
and cyclophosphamide program...
Clearly, a risk reduction of 20%-25% in disease-free survival with one regimen relative to the other would provide differentiating information based on efficacy. This study will be powered to demonstrate those differences if they are present.
However, if the regimens do not differ in relative efficacy by that magnitude, the major determinate of clinical utility would be relative toxicity, and direct comparison in a randomized trial will provide that information...
Although the TAC and DD AC![]() P regimens
have improved treatment outcome, unfortunately
women treated with either regimen still develop
local, regional, and systemic disease recurrence.
This reality provides a compelling reason to
continue efforts to further improve therapy for
node-positive breast cancer.
P regimens
have improved treatment outcome, unfortunately
women treated with either regimen still develop
local, regional, and systemic disease recurrence.
This reality provides a compelling reason to
continue efforts to further improve therapy for
node-positive breast cancer.
One potential advantage of DD AC![]() P is that its
reported toxicity profile provides opportunity for
incorporating a fourth chemotherapeutic agent
into the program by adding it to the paclitaxel
sequence.
P is that its
reported toxicity profile provides opportunity for
incorporating a fourth chemotherapeutic agent
into the program by adding it to the paclitaxel
sequence.
The anti-metabolite gemcitabine has shown promise in combination with paclitaxel for treatment of metastatic breast cancer using various schedules, including every 2-week dosing intervals.
Correlative Science Program
The NSABP has an ongoing correlative science program that is attempting to identify prognostic factors for node-positive patients treated with 4 cycles of AC, as well as predictive factors for benefit from additional chemotherapeutic agents such as paclitaxel, docetaxel, and gemcitabine.
To support this important work, tissue block submission will be mandatory for all patients who have given consent for tissue submission so complete tissue arrays can be developed for this trial.
COMMENTS FROM BREAST CANCER INVESTIGATORS
![]() The ECOG-E2104 pilot adjuvant trial is critically
important because it will evaluate adding
bevacizumab to an anthracycline-based treatment
regimen.
The ECOG-E2104 pilot adjuvant trial is critically
important because it will evaluate adding
bevacizumab to an anthracycline-based treatment
regimen.
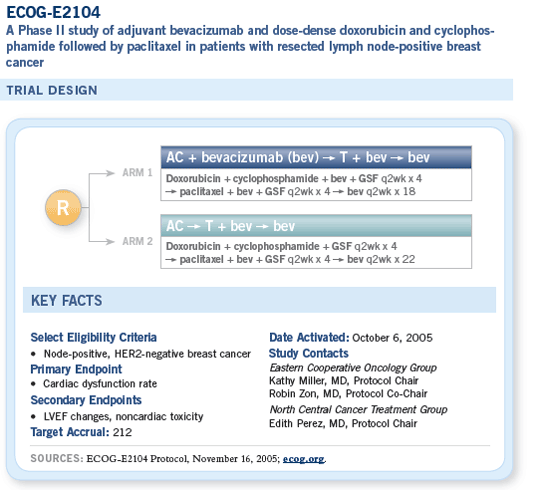
The trial will enroll 212 patients, and the chemotherapy regimen is dose-dense AC followed by paclitaxel. ECOG-E2104 is observing two different cohorts.
The first cohort receives bevacizumab with the anthracycline and throughout therapy. The second cohort receives bevacizumab only with paclitaxel, and this is our backup if we do see cardiac toxicity issues with the combined administration. Hence, we’ll have safety data with both strategies.
The full adjuvant trial will use a slightly different chemotherapy backbone that won’t require growth factors. We will be using AC on an every three-week basis followed by weekly paclitaxel. I wanted to use a weekly taxane regimen because the biggest support for moving this into the adjuvant setting is the data from ECOG-E2100, which used a weekly taxane schedule.
The proposed full adjuvant trial (ECOG-E5103) has three arms, on which everybody receives the same chemotherapy. Patients in arm A receive no bevacizumab. Those in arm B receive six months of bevacizumab, concurrently with chemotherapy, and those in arm C receive 12 months of bevacizumab, six months with chemotherapy and an additional six months of maintenance.
The first six months of therapy are blinded and placebo controlled. At the end of the chemotherapy treatment, patients and their physicians will be told to which arm they have been assigned and whether they’re continuing bevacizumab for an additional six months.
With regard to the signal seen in E2100, we expect much greater activity in the adjuvant setting, and recent laboratory data suggest that we’re likely to see it. First-line chemotherapy for metastatic disease is fairly late in the natural history of breast cancer.
Although the patients in the E2100 trial hadn’t received chemotherapy for metastatic disease, two thirds of them had received adjuvant chemotherapy, and 18 percent had received a taxane. These were not chemotherapy-naïve patients. They were much more advanced than the patients enrolled a decade ago in trials of first-line chemotherapy for metastatic disease.
— Kathy D Miller, MD
![]() We know relatively little about bevacizumab
and the heart, and this, of course, remains an
issue. Certainly, with trastuzumab, clinicians and
laboratory scientists were absolutely astonished
when trastuzumab was found to increase congestive
cardiomyopathy with anthracycline-based
chemotherapy, and that was due to a lack of
understanding of the biology of the disease.
We know relatively little about bevacizumab
and the heart, and this, of course, remains an
issue. Certainly, with trastuzumab, clinicians and
laboratory scientists were absolutely astonished
when trastuzumab was found to increase congestive
cardiomyopathy with anthracycline-based
chemotherapy, and that was due to a lack of
understanding of the biology of the disease.
We have a number of small studies, and these typically involve 20 to 40 patients, where patients have received bevacizumab in combination with an anthracycline or with an anthracenedione, and in a number of these studies, there is just barely a hint of some increased rate of congestive cardiomyopathy.
The problem with these studies is that they tend to be in populations of patients who are already at increased risk for congestive cardiomyopathy in that, in addition to anthracyclines, they frequently will have undergone prolonged use of anthracyclines up to a larger dose in the metastatic setting than we would ever use in the adjuvant setting. These patients frequently will have undergone left chest wall irradiation. So we’re simply not sure whether or not this is a true signal or a false one.
ECOG-E2104 was designed to answer this question. This is a pilot adjuvant trial in which patients in the first part of the trial receive doxorubicin and cyclophosphamide followed by paclitaxel, administered in a dose-dense fashion, and patients receive bevacizumab from the beginning with careful cardiac monitoring before, during and following the year’s period of bevacizumab.
The second part of this trial is only evaluating bevacizumab in combination with paclitaxel but, again, with the same careful cardiac monitoring.
The trial is not powered to pick up tiny signals. It’s powered to pick up fairly significant signals. But the first half of the trial has completed accrual, and we expect to have at least some initial data from this in a fairly short time period.
The National Cancer Institute will be taking data from this trial and combining it with two smaller Phase II trials in which an anthracycline has been combined with bevacizumab to get some sense of the rate of congestive cardiomyopathy.
If that rate is significant, then what we would do in the large randomized trial would be to give bevacizumab solely with the paclitaxel. We’re hoping, of course, that won’t be the case.
— George W Sledge Jr, MD
![]() We’re anxiously awaiting the safety analysis of
ECOG trial E2104. The primary endpoint for that
study is cardiac safety. Adjuvant bevacizumab
hinges on the demonstration of safety because
many of these patients will be cured of their
disease.
We’re anxiously awaiting the safety analysis of
ECOG trial E2104. The primary endpoint for that
study is cardiac safety. Adjuvant bevacizumab
hinges on the demonstration of safety because
many of these patients will be cured of their
disease.
Clinicians will be loath to put patients through anything that might substantially increase their risks of complications. So we’ll have to wait and see what that data set looks like and what our Phase II metastatic bevacizumab/trastuzumab combination looks like before we can design the next adjuvant trial that might incorporate both.
Practicing clinicians should probably wait on the sidelines to see these safety data sets before embarking on any of these combinations. Serious concern for safety exists with these types of combinations, and clinicians shouldn’t do anything off protocol in the absence of the Phase II data.
— Mark D Pegram, MD
![]() It’s certainly possible that adding bevacizumab
to an anthracycline might improve the antitumor
effect. Most of us believe that bevacizumab
is a drug that’s acting pretty specifically on
the VEGF target, more specifically than the
small molecules, like sunitinib, which have
multiple targets.
It’s certainly possible that adding bevacizumab
to an anthracycline might improve the antitumor
effect. Most of us believe that bevacizumab
is a drug that’s acting pretty specifically on
the VEGF target, more specifically than the
small molecules, like sunitinib, which have
multiple targets.
Certainly VEGF is playing a role as a component of a survival signal that allows cells to survive a variety of drugs, including the anthracyclines.
I believe the strongest rationale for potential synergy is emphasized when you’re administering a chemotherapy program, either weekly paclitaxel or our so-called metronomic version of AC, for which you have reason to believe that the program itself is anti-angiogenic, because then you would expect to have a second hit on the same pathway through a different mechanism of action.
— Robert B Livingston, MD
![]() Certainly, concern remains about long-term
toxicity associated with bevacizumab. For that
matter, some concern exists about short-term
toxicity. So within the cooperative groups, the
sense is “to wade in” but wade in not so rapidly.
Certainly, concern remains about long-term
toxicity associated with bevacizumab. For that
matter, some concern exists about short-term
toxicity. So within the cooperative groups, the
sense is “to wade in” but wade in not so rapidly.
A large ECOG pilot trial is evaluating bevacizumab
administered in addition to AC![]() T,
primarily with toxicity endpoints in the adjuvant
setting. Assuming that pilot study is successful
— meaning that it does not show undue toxicity
— a large, randomized trial will compare AC
T,
primarily with toxicity endpoints in the adjuvant
setting. Assuming that pilot study is successful
— meaning that it does not show undue toxicity
— a large, randomized trial will compare AC![]() T
to AC
T
to AC![]() T with bevacizumab.
What remains unclear is the timing of the bevacizumab
— whether it will be administered with all
of the chemotherapy or just with the taxane.
T with bevacizumab.
What remains unclear is the timing of the bevacizumab
— whether it will be administered with all
of the chemotherapy or just with the taxane.
One important aspect of the trial is that not everyone will be assigned to the same duration of bevacizumab. Given concerns about toxicity and about cost issues, an arm of the trial will receive a relatively short duration of bevacizumab.
Finally, a plan is also in place to evaluate bevacizumab within the cooperative groups in the preoperative setting to see if we can better characterize which patients and which tumors derive benefit, because, although we’ve been unsuccessful in the past with the ability to biopsy and rebiopsy, in the preoperative setting that may be more feasible.
— Eric P Winer, MD
SUPPORTING PROTOCOL INFORMATION
The optimal scheduling for administration of
doxorubicin and cyclophosphamide followed by
paclitaxel (AC![]() T) was investigated in CALGB-
9741. This trial enrolled 2,005 patients in a 2 x
2 factorial design to compare sequential versus
concurrent administration of doxorubicin and
cyclophosphamide and two 21 day versus 14 day
treatment intervals.
T) was investigated in CALGB-
9741. This trial enrolled 2,005 patients in a 2 x
2 factorial design to compare sequential versus
concurrent administration of doxorubicin and
cyclophosphamide and two 21 day versus 14 day
treatment intervals.
Though there was no difference in event rates between sequential and concurrent administration of doxorubicin and cyclophosphamide (p = 0.67), use of the 14 day (dose dense) schedule improved both disease-free and overall survival (p = 0.013).
The improvement in overall survival with the dose dense schedule persisted in a multivariate Cox proportional hazards model after adjusting for standard baseline covariates (risk ratio 1.45; p = 0.014). ...
Over the last two decades substantial laboratory and indirect clinical evidence has accumulated to support the central role of angiogenesis in breast cancer progression. This nascent vascular network provides a novel opportunity for therapy. However, as tumors progress, increasing numbers of pro-angiogenic peptides are produced.
As such, the most successful clinical application of angiogenesis inhibitors is likely to be in the adjuvant setting. ...Proof of this concept will require large, prospective randomized trials in the adjuvant setting.
This trial will provide the safety and feasibility data in a selected group of patients with early stage disease needed to justify a full-scale phase III adjuvant trial. ...
Bevacizumab has been studied in at least 3,500 patients in a number of Phase I, II, and III clinical trials in a number of tumor types, including colorectal, breast, lung, and renal carcinoma. In the Phase I and II clinical trials, four potential bevacizumab-associated safety signals were identified: hypertension, proteinuria, thromboembolic events, and hemorrhage.
Completed Phase II and Phase III studies of bevacizumab have further defined the safety profile of this agent in patients with metastatic malignancies.
Also during the Phase III trials, three new possible bevacizumab-associated safety signals were identified: congestive heart failure (CHF) in patients who had been exposed to anthracyclines, gastrointestinal perforations, and wound healing complications.
COMMENTS FROM BREAST CANCER INVESTIGATORS
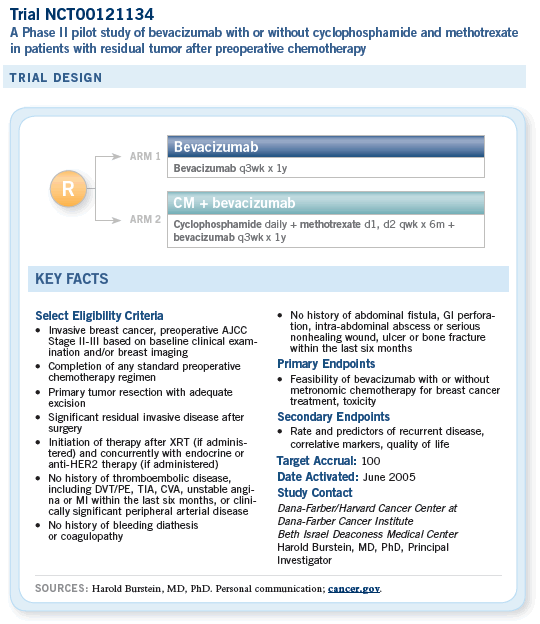
![]() The study that we have activated now at Dana-Farber and Indiana University with my good
friends Kathy Miller and George Sledge is a pilot
study of bevacizumab in the adjuvant setting. The
patient population has had preoperative chemotherapy
for breast cancer and has residual cancer
at the time of their surgery.
The study that we have activated now at Dana-Farber and Indiana University with my good
friends Kathy Miller and George Sledge is a pilot
study of bevacizumab in the adjuvant setting. The
patient population has had preoperative chemotherapy
for breast cancer and has residual cancer
at the time of their surgery.
Those patients will be offered one year of bevacizumab therapy to see if it’s feasible, and then a second cohort of the same type of patients will be offered one year of bevacizumab and six months of metronomic chemotherapy.
We chose this patient population for a couple of specific reasons. First, we know that women who have residual disease after preoperative chemotherapy constitute a patient population at high risk, for whom there is no standard treatment.
Second, these women have tumors that by definition have some resistance to chemotherapy. So instead of just treating them with more chemotherapy, we thought it would be interesting to bring in a biologic agent.
When we find residual disease after neoadjuvant chemotherapy, clinicians are tempted to offer more chemotherapy, which is understandable. However, there are many reasons to believe it’s not going to be effective.
First, no data suggest that more chemotherapy is beneficial in this setting. Second, there’s reason to believe that women with tumors like that have disease that is more or less intrinsically resistant to chemotherapy.
The rationale for the bevacizumab-alone arm was also twofold. First, we don’t know that bevacizumab alone would not be effective.
Of course, the adjuvant trials that are going to answer this question will ultimately be large cooperative group studies of chemotherapy with or without bevacizumab.
Second, we wanted to see if it would be safe to give six to 12 months of bevacizumab in the adjuvant setting. Bevacizumab alone has the advantage of being better tolerated, so when you start discussing extended periods of therapy, it probably is more feasible.
We also have some handsome correlative studies built into this trial. These studies take advantage of the proteomics research for which Indiana University is well known and evaluate some other markers of tumor recurrence and endothelial cell biology in which our group is interested.
— Harold J Burstein, MD, PhD
![]() The use of antiangiogenics as adjuvant therapy
has its own potential barriers. The toxicity of
chronic antiangiogenic therapy remains largely
unexplored, as is the toxicity of combinations of
chemotherapy with antiangiogenic therapy.
The use of antiangiogenics as adjuvant therapy
has its own potential barriers. The toxicity of
chronic antiangiogenic therapy remains largely
unexplored, as is the toxicity of combinations of
chemotherapy with antiangiogenic therapy.
Although intuitively the impact of angiogenesis inhibition is expected to be greatest in patients with micrometastatic disease, proof of this concept will require commitment of substantial human and financial resources to a randomized adjuvant trial.
— Bryan P Schneider, MD, Kathy D Miller, MD.
J Clin Oncol 2005;23(8):1782-90.
![]() Conventional cytotoxic chemotherapeutic drugs
treat cancer either by direct killing or by inhibition
of growth of cycling tumor cells. In addition,
evidence suggests that cytotoxic agents may
inhibit tumor growth through an antiangiogenic
mechanism.
Conventional cytotoxic chemotherapeutic drugs
treat cancer either by direct killing or by inhibition
of growth of cycling tumor cells. In addition,
evidence suggests that cytotoxic agents may
inhibit tumor growth through an antiangiogenic
mechanism.
“Metronomic” or frequent continuous administration of the same chemotherapeutic agents at lower doses may optimize their antiangiogenic properties.
The effectiveness of metronomic chemotherapy regimens can be improved significantly by concurrent administration of antiangiogenic, endothelial-specific drugs.
Preclinical studies have shown that integrating chemotherapy with antiangiogenic drugs can improve efficacy and circumvent the toxicity and drug resistance associated with standard or high-dose chemotherapy.
Preliminary clinical studies have shown similar results.
— Harman P Kaur, MD, Thomas G Budd, MD.
Curr Oncol Rep 2004;6(1):49-52.
![]() An intriguing hypothesis is the possibility of
synergy between anti-VEGF agents and chemotherapy,
with respect to the inhibition of angiogenesis.
An intriguing hypothesis is the possibility of
synergy between anti-VEGF agents and chemotherapy,
with respect to the inhibition of angiogenesis.
Chemotherapy likely targets dividing endothelial cells found in newly forming blood vessels; however, these cells are relatively slow growing, and conventional cycle lengths may allow for repair and recovery from some of the chemotherapy-induced damage.
Researchers have devised antiangiogenic or metronomic chemotherapy dosing schedules in order to apply continuous pressure on the newly forming tumor vasculature and possibly overcome acquired chemotherapy resistance.
This approach involves either continuous chemotherapy infusion or regular, frequent chemotherapy administration, generally with lower chemotherapy doses to avoid excess toxicity.
Indeed, there is evidence in humans that this approach overcomes drug resistance, as patients resistant to conventional taxane therapy have been found to respond subsequently to lower-dose weekly treatment.
— Clifford Hudis, MD.
Oncology (Williston Park) 2005;
19(4 Suppl 3):26-31.
Editor’s Note:
Let’s get this thing done.
Prologue:
The adjuvant trastuzumab clinical research to
practice model
- Select publications
Breast Cancer Clinical Trials:
Neoadjuvant Therapy