
 |
||||||||

| Tracks 1-15 | ||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 2, 4
![]() DR GEYER: It was a Phase III randomized trial comparing capecitabine alone
to capecitabine in combination with lapatinib — a dual EGFR and HER2
tyrosine kinase inhibitor — in patients with progressive metastatic HER2-positive breast cancer. All the women had received previous therapy with an
anthracycline and a taxane in either the adjuvant or metastatic setting. They
also received trastuzumab in the metastatic setting (Geyer 2006).
DR GEYER: It was a Phase III randomized trial comparing capecitabine alone
to capecitabine in combination with lapatinib — a dual EGFR and HER2
tyrosine kinase inhibitor — in patients with progressive metastatic HER2-positive breast cancer. All the women had received previous therapy with an
anthracycline and a taxane in either the adjuvant or metastatic setting. They
also received trastuzumab in the metastatic setting (Geyer 2006).
Patients had to have tumors with IHC 3+ or amplification by FISH to be eligible, and they had to have a normal ejection fraction, measurable disease and a reasonably good performance status (ECOG 0 or 1) even though they were pretreated.
The patients were randomly assigned to capecitabine at a total dose of 2,500 mg/m2 per day or capecitabine at 2,000 mg/m2 per day with lapatinib at 1,250 mg per day. Lapatinib was administered on a continuous basis and capecitabine on days one through 14 every 21 days (Geyer 2006).
The study was set up originally to look for an improvement in median time to progression from three months to 4.5 months. Because the group was pretreated and had HER2-positive disease, it was thought that those receiving capecitabine would probably have a median time to progression of about three months, and a 4.5-month median time to progression was felt to be a reasonable improvement. Overall survival was also evaluated.
The original sample size was 528 patients, but like all large Phase III studies it included an interim monitoring plan. The final evaluation for time to progression was supposed to occur with 266 events, and the interim analysis would occur at 133 events. Because this was an open-label trial, a blinded independent review committee (IRC) was to read all the films.
As of November 15, 2005, 114 events were recorded and 321 patients were enrolled. The 321 patients’ films were reviewed, and the IRC determined that 114 events met the criteria for the study. It went to the Independent Data Monitoring Committee (IDMC), where it was determined that the study had crossed the O’Brien-Fleming boundaries for early reporting by a wide margin (Geyer 2006).
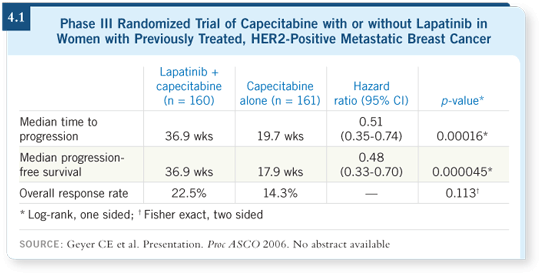
The capecitabine-alone group did better than anticipated. Instead of three months, the median time to progression was about 4.5 months. The group receiving capecitabine and lapatinib had a median time to progression of about 8.5 months. The median time to progression was nearly doubled (Geyer 2006; [4.1]).
Also, the overall response rate was improved from roughly 14 to 22 percent (4.1). Clearly it was an active regimen. The IDMC assessed the safety data and found minimal Grade IV toxicities (Geyer 2006).
It was a well-tolerated regimen, which is surprising because concern had been raised about significant synergy in terms of toxicity, but this did not arise. Based on the efficacy and the safety, the IDMC made the recommendation that the accrual be closed, the results announced and the patients who were still receiving capecitabine alone be offered lapatinib (Geyer 2006).
![]() DR LOVE: If lapatinib were available and you were to see a patient who has
progressed on trastuzumab, how would you treat that patient?
DR LOVE: If lapatinib were available and you were to see a patient who has
progressed on trastuzumab, how would you treat that patient?
![]() DR GEYER: I think a patient who meets the eligibility criteria of the trial
certainly should be offered lapatinib, when it becomes available.
DR GEYER: I think a patient who meets the eligibility criteria of the trial
certainly should be offered lapatinib, when it becomes available.
Track 7
![]() DR GEYER: We are currently working on a straightforward concept evaluating
trastuzumab versus lapatinib versus the combination using an AC
followed by weekly paclitaxel template as neoadjuvant therapy. All the patients
will receive that basic chemotherapy regimen, and the HER2 blockade will
start with paclitaxel.
DR GEYER: We are currently working on a straightforward concept evaluating
trastuzumab versus lapatinib versus the combination using an AC
followed by weekly paclitaxel template as neoadjuvant therapy. All the patients
will receive that basic chemotherapy regimen, and the HER2 blockade will
start with paclitaxel.
Then the patients will have surgery to determine the pathologic complete response rate. After surgery, all the patients will receive trastuzumab for one year. They will be receiving standard therapy with trastuzumab, but we will obtain baseline tissue and do the correlative work to see if we can determine which patients might do better with each of the drugs individually or in combination.
Track 8
![]() DR GEYER: We are committed to collaborating with Dennis Slamon and the
BCIRG jointly on that concept. We have been waiting for their pilot data
evaluating the combination of bevacizumab and trastuzumab as front-line
therapy for patients with HER2-positive disease.
DR GEYER: We are committed to collaborating with Dennis Slamon and the
BCIRG jointly on that concept. We have been waiting for their pilot data
evaluating the combination of bevacizumab and trastuzumab as front-line
therapy for patients with HER2-positive disease.
The trial is progressing well, and from what they have been able to share, it looks as if this is something we definitely will be pursuing.
![]() DR LOVE: What do we know about the potential synergy between bevacizumab
and trastuzumab?
DR LOVE: What do we know about the potential synergy between bevacizumab
and trastuzumab?
![]() DR GEYER: When patients’ tumors have HER2 amplification, a high
percentage — about three quarters of the patients — also have upregulation of
VEGF. Those patients do not do well when treated with chemotherapy alone;
they have a strikingly poor outcome.
DR GEYER: When patients’ tumors have HER2 amplification, a high
percentage — about three quarters of the patients — also have upregulation of
VEGF. Those patients do not do well when treated with chemotherapy alone;
they have a strikingly poor outcome.
The assumption is that something is mechanistically driving the cancer, and if you shut down both of those pathways, you will improve outcomes. Preclinical models look very strong, and they were the justification for taking this into a clinical trial.
Track 9
![]() DR GEYER: The exciting thing about the adjuvant trastuzumab data has been
that no matter how you use it, patients derive a substantial benefit. Small
differences probably occur among the different ways of using it, which we
can’t definitively address because the trials weren’t designed that way, but it’s
clear that trastuzumab is the most important element of therapy for a patient
with HER2-positive breast cancer.
DR GEYER: The exciting thing about the adjuvant trastuzumab data has been
that no matter how you use it, patients derive a substantial benefit. Small
differences probably occur among the different ways of using it, which we
can’t definitively address because the trials weren’t designed that way, but it’s
clear that trastuzumab is the most important element of therapy for a patient
with HER2-positive breast cancer.
The fact that a woman doesn’t meet the eligibility criteria of the original trials doesn’t mean that she shouldn’t receive trastuzumab. I believe she should receive the safest regimen. TCH (docetaxel/carboplatin/trastuzumab) certainly has low cardiac toxicity, but TCH is not a gentler regimen for an elderly woman. It is a fairly rigorous program in and of itself, though the cardiac toxicity is less.
I believe the weekly carboplatin/paclitaxel/trastuzumab that we use for metastatic disease is active and well tolerated. Those are the substitutions I believe would be reasonable to consider for an elderly patient, if you felt you needed to use chemotherapy.
Can you use only trastuzumab or hormone therapy? I’m sure you can. You have to use your clinical judgment. Trastuzumab is active without chemotherapy; there is no question about that. If I were going to use trastuzumab, I would like to use some kind of chemotherapy, maybe just four cycles á la the HERA trial (Piccart-Gebhart 2005).
Track 11
![]() DR GEYER: For me, the precedent for cardiac monitoring has been set by
the adjuvant trials. The plan was a reasonable one: Check imaging halfway
through the chemotherapy, check it at the end of chemotherapy and then check it three months later. It made sense for the trial, and I believe it makes
sense for the clinic.
DR GEYER: For me, the precedent for cardiac monitoring has been set by
the adjuvant trials. The plan was a reasonable one: Check imaging halfway
through the chemotherapy, check it at the end of chemotherapy and then check it three months later. It made sense for the trial, and I believe it makes
sense for the clinic.
In NSABP-B-31 and NCCTG-N9831, we stopped the drug in a significant number of patients — about 15 percent of the patients had asymptomatic declines in LVEF (Romond 2005). We don’t know that we would have seen a higher rate of clinical heart failure if we had continued to treat them, but it’s a reasonable assumption.
Track 12
![]() DR GEYER: That is NSABP-B-40 (Figure 1, page 5), which was originally
going to be a three-arm study evaluating sequential AC followed by either
docetaxel alone, docetaxel with capecitabine or docetaxel with gemcitabine.
We were about to open the trial but decided to modify it to incorporate
bevacizumab. With that, we reconfigured the study to move the taxane ahead
of the AC, which is the reversal of the usual order.
DR GEYER: That is NSABP-B-40 (Figure 1, page 5), which was originally
going to be a three-arm study evaluating sequential AC followed by either
docetaxel alone, docetaxel with capecitabine or docetaxel with gemcitabine.
We were about to open the trial but decided to modify it to incorporate
bevacizumab. With that, we reconfigured the study to move the taxane ahead
of the AC, which is the reversal of the usual order.
Our thinking was twofold. First, the data for bevacizumab in breast cancer were with a taxane. Hence we wanted to administer the two together as much as possible. Second, the possibility of increased cardiac toxicity for the anthracyclines with bevacizumab has been a concern.
More and more, it’s looking as if that isn’t going to be an issue, but because we have to stop bevacizumab a couple of cycles before surgery, it also makes sense from that perspective.
Track 13
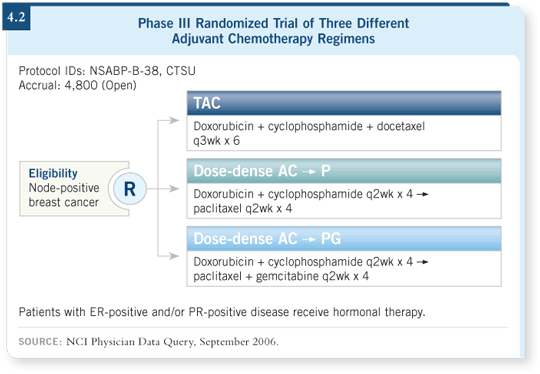
![]() DR GEYER: The trial has three arms (4.2). In a sense, we have two control
groups. We have the docetaxel control arm using the TAC regimen from the
BCIRG study (Martin 2005). Our paclitaxel control arm is the dose-dense
regimen (Citron 2003). The third arm adds gemcitabine to the dose-dense
paclitaxel portion of the regimen.
DR GEYER: The trial has three arms (4.2). In a sense, we have two control
groups. We have the docetaxel control arm using the TAC regimen from the
BCIRG study (Martin 2005). Our paclitaxel control arm is the dose-dense
regimen (Citron 2003). The third arm adds gemcitabine to the dose-dense
paclitaxel portion of the regimen.
The improvement with dose-dense therapy seemed to be largely confined to the patients with ER-negative disease. Does that mean dose-dense therapy isn’t right for a patient with ER-positive disease? My view is that dose-dense therapy isn’t less effective than every three-week therapy.
Clearly the big step is among patients with ER-negative disease, but it still has advantages in that the therapy is finished sooner. We still consider it a viable option.
In terms of adjuvant chemotherapy for patients with node-positive disease, about all you can say is that they probably should receive an anthracycline, cyclophosphamide and a taxane. The recipes are all over the place. This does allow a lot of flexibility for physicians and patients to make selections for offprotocol therapy.
When you have an active protocol that is evaluating an optimal docetaxel regimen, an optimal paclitaxel regimen and a possible improvement, you have no reason to reconfigure that study.
I believe the dose-dense arm is a very reasonable treatment for patients with ER-positive, node-positive disease.
Editor’s Note:
Reality check
Interviews
Marc E Lippman, MD
- Select publications
C Kent Osborne, MD
- Select publications
I Craig Henderson, MD
- Select publications
Charles E Geyer Jr, MD
- Select publications
Frank A Vicini, MD
- Select publications
Miami Beach Cancer Conference Tumor Panel Discussion on Systemic Therapy of Metastatic Disease
- Select publications