
 |
||||||||

| Tracks 1-11 | ||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
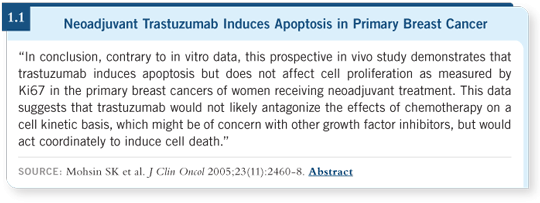
![]() DR LIPPMAN: Some conflicting data exist on the mechanisms of action of
trastuzumab. One thing that seems fairly certain is that it interferes with the
signaling of the EGF receptor family on the cell surface.
DR LIPPMAN: Some conflicting data exist on the mechanisms of action of
trastuzumab. One thing that seems fairly certain is that it interferes with the
signaling of the EGF receptor family on the cell surface.
Trastuzumab is an antibody to HER2. By interfering with that receptor, it blocks the intracellular signaling from that receptor, which is, in effect, a growth factor signal to the cell. Therefore, the cell is unable to continue its normal proliferative process and commonly undergoes apoptotic cell death.
Track 6
![]() DR LIPPMAN: Almost 30 years ago, we published, in The New England Journal
of Medicine, that patients with ER-negative disease responded more frequently
to chemotherapy (Lippman 1978) than patients with ER-positive disease.
Those data have been replicated in the meta-analyses conducted in England by
Sir Richard Peto and his collaborators (EBCTCG 2005).
DR LIPPMAN: Almost 30 years ago, we published, in The New England Journal
of Medicine, that patients with ER-negative disease responded more frequently
to chemotherapy (Lippman 1978) than patients with ER-positive disease.
Those data have been replicated in the meta-analyses conducted in England by
Sir Richard Peto and his collaborators (EBCTCG 2005).
The clue as to why that occurs is obtained if you observe recurrence rates for women with breast cancer as a function of whether their disease is ER-positive or ER-negative. It is commonly said, but that doesn’t necessarily make it the truth, that having ER-positive disease is a good prognostic factor. The data show — and this has now been shown several times — that early on, if your disease is ER-positive, your relapse rates are lower.
Over time, the patients with ER-negative disease who relapse at a higher rate initially stop relapsing, perhaps because most of the ones with bad prognoses have already died, whereas the patients with ER-positive disease continue to relapse, and those lines actually cross. At about 10 to 15 years, you’re worse off having ER-positive than ER-negative disease.
Biologically, that probably means ER-positive tumors are growing more slowly. Therefore, the whole story of why chemotherapy works a little better is probably based on the fact that ER-negative tumors have a slightly higher cycling rate and are faster growing. A wealth of data suggests they’re a little more sensitive to chemotherapy.
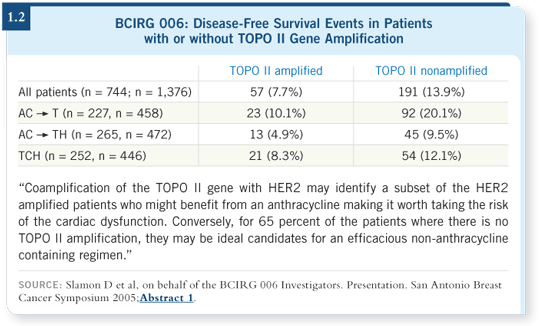
Tracks 7, 8
![]() DR LIPPMAN: These were very exciting data, which I hope are substantiated. It makes biological sense — TOPO II is a target for doxorubicin. That
would potentially explain which subsets of patients gained particular advantage
from the doxorubicin combinations compared to the platinum combinations
(Slamon 2005; [1.2]).
DR LIPPMAN: These were very exciting data, which I hope are substantiated. It makes biological sense — TOPO II is a target for doxorubicin. That
would potentially explain which subsets of patients gained particular advantage
from the doxorubicin combinations compared to the platinum combinations
(Slamon 2005; [1.2]).
I’m not ready to draw the conclusion that Professor Slamon seemed to want to draw, which is that in those patients who did not overexpress TOPO II, the use of a nondoxorubicin-containing combination was as efficacious (Slamon 2005).
That may be true, but I’m not there yet. I believe we need more analysis. Given the additional cardiac risks of using trastuzumab with doxorubicin, particularly in older women, it would be nice to have a less cardiotoxic regimen to use.
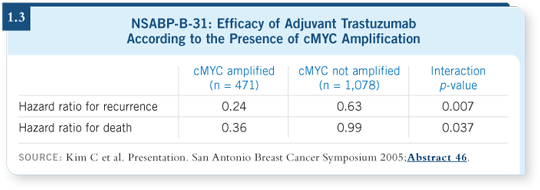
In that same regard, I found the data Soon Paik presented from the NSABP on cMYC overexpression (Kim 2005; [1.3]) extremely exciting and, once again, biologically plausible.
cMYC is an oncogene that is generally upregulated when cells are stimulated to grow; it is part of the growth response. When cMYC is overexpressed in tumors, it can transform other cells to become cancerous, and it is clearly overexpressed in about 20 to 25 percent of human breast cancer cases.
The question is, why is it that many patients with tumors that unquestionably overexpress HER2 do not respond to trastuzumab? Even in previously untreated patients, the response rates are only about 35 percent.
Dr Paik’s data showed rather conclusively that only in those patients whose tumors coexpressed cMYC and HER2 was a response to trastuzumab seen (Kim 2005; [1.3]). Those data must be replicated, but if that is the case, this observation would be tremendously insightful.
Editor’s Note:
Reality check
Interviews
Marc E Lippman, MD
- Select publications
C Kent Osborne, MD
- Select publications
I Craig Henderson, MD
- Select publications
Charles E Geyer Jr, MD
- Select publications
Frank A Vicini, MD
- Select publications
Miami Beach Cancer Conference Tumor Panel Discussion on Systemic Therapy of Metastatic Disease
- Select publications