
 |
||||||||

| Tracks 1-11 | ||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3
![]() DR HENDERSON: The evidence is pretty clear that TOPO II makes sense
scientifically. We began talking about it more than a decade ago. It’s particularly
interesting because TOPO II is on the same chromosome as HER2, and
in the early papers we thought there was a correlation between the use of
doxorubicin and HER2.
DR HENDERSON: The evidence is pretty clear that TOPO II makes sense
scientifically. We began talking about it more than a decade ago. It’s particularly
interesting because TOPO II is on the same chromosome as HER2, and
in the early papers we thought there was a correlation between the use of
doxorubicin and HER2.
I don’t believe that has really held up. Certainly, when Dan Hayes presented the data from CALGB-9344 at ASCO 2006 we didn’t see a correlation between HER2 expression and doxorubicin dose (Hayes 2006).
![]() DR LOVE: If a clinician had the results of a TOPO II assay for a patient, should they be considered when deciding on therapy and whether an anthracycline
is necessary?
DR LOVE: If a clinician had the results of a TOPO II assay for a patient, should they be considered when deciding on therapy and whether an anthracycline
is necessary?
![]() DR HENDERSON: I believe anthracyclines are so powerful and so valuable in
the treatment of breast cancer that I would be hesitant to leave out doxorubicin
until we had compelling data that a particular group of patients received
no benefit from it.
DR HENDERSON: I believe anthracyclines are so powerful and so valuable in
the treatment of breast cancer that I would be hesitant to leave out doxorubicin
until we had compelling data that a particular group of patients received
no benefit from it.
It’s similar to the way we view estrogen receptor status and chemotherapy. We know that patients with ER-positive disease derive less benefit from chemotherapy than those with ER-negative breast cancer, but it’s not an all-or-none phenomenon.
I believe the same principle applies here. When will you be comfortable enough to leave out a powerful drug? As good as the taxanes are — and I am enthusiastic about them — I don’t believe they are any better than the anthracyclines in the treatment of breast cancer.
Track 4
![]() DR HENDERSON: I am enthusiastic about nab paclitaxel. I have a bias in that I
was very involved in the development of doxorubicin HCL liposome injection
and it, like nab paclitaxel, has a delivery system that increases the amount of
drug that actually reaches the tumor.
DR HENDERSON: I am enthusiastic about nab paclitaxel. I have a bias in that I
was very involved in the development of doxorubicin HCL liposome injection
and it, like nab paclitaxel, has a delivery system that increases the amount of
drug that actually reaches the tumor.
The issue of dose of chemotherapy has been a complicated one in cancer. When we examine dose in animal models, we clearly see a dose effect, and in leukemia we see an advantage with higher doses. Almost every oncologist has been taught as part of his or her earliest training that dose is a critical factor. However, in most dose studies it’s difficult to demonstrate that dose makes a lot of difference, high-dose chemotherapy in bone marrow transplant being a case in point. I believe the reason we have been unable to show that dose is so important is that we are examining the dose we administer rather than the dose that reaches the tumor.
With a delivery system, you change the distribution of drug so that less goes to the normal tissue and more — a higher dose — reaches the tumor itself. That’s what happens with doxorubicin HCL liposome injection and nab paclitaxel. In both cases we can show that elegantly in preclinical models. Showing that in the human, of course, is more difficult because it’s not so easy to biopsy a tumor and measure the drug level.
We know that we can administer higher doses. In CALGB-9342, which studied paclitaxel doses of 175 mg/m2, 210 mg/m2 and 250 mg/m2 in patients with metastatic breast cancer, we saw no significant effect from escalating the paclitaxel dose (Winer 2004). However, there was some marginal effect from the higher doses and a suggestion of a longer time to tumor progression.
In fact, some of the analyses reached statistical significance as an endpoint. I believe with nab paclitaxel we are seeing that we can give higher doses and that patients tolerate higher doses.
In the preclinical models, mice tolerate higher doses of nab paclitaxel than paclitaxel delivered in Cremophor®. In addition, because of the way the albumin interacts with the paclitaxel, higher doses were delivered to the tumor.
I believe that’s why they were able to show a significantly better outcome with nab paclitaxel. It’s an interesting step forward.
Track 6
![]() DR HENDERSON: SPARC is an interesting observation. What’s important
about SPARC is that albumin appears to bind to it and SPARC is overexpressed
in a number of different tumors (3.1). It’s a protein that was first
described 10 to 15 years ago. A lot of preclinical work has been conducted on
this, and it’s a story that makes sense.
DR HENDERSON: SPARC is an interesting observation. What’s important
about SPARC is that albumin appears to bind to it and SPARC is overexpressed
in a number of different tumors (3.1). It’s a protein that was first
described 10 to 15 years ago. A lot of preclinical work has been conducted on
this, and it’s a story that makes sense.
You are dealing with a larger particle with nab paclitaxel, and a larger particle can go through the gaps that occur in tumor tissue because the vasculature is leaky, whereas the junctions between blood vessel cells in normal tissues are tighter and won’t allow these big particles to go through.
That’s one reason why a higher level of paclitaxel is delivered into the tumor with nab paclitaxel. A second reason is the binding to the albumin-binding sites in the vessels, which helps take a larger amount of the paclitaxel-bound albumin into the tumor.

Third, you have the binding to SPARC, an attraction to the tumor itself. All three of these reasons seem to explain why you get a better response to nab paclitaxel than you do to Cremophor-based paclitaxel.
![]() DR LOVE: Is it possible to do an assay of SPARC, and could that, theoretically,
be used to select therapies?
DR LOVE: Is it possible to do an assay of SPARC, and could that, theoretically,
be used to select therapies?
![]() DR HENDERSON: Theoretically, I believe it could.
DR HENDERSON: Theoretically, I believe it could.
Track 7
![]() DR HENDERSON: It is an irony because intuitively you would think that
bevacizumab would have exactly the opposite effect. In other words, we have
always known that necrosis is one of the problems with delivering chemotherapy
to the inside of the tumor. Tumors don’t have a good vascular system,
so there’s not very much oxygen in there. Therefore, radiation therapy isn’t
as effective, and chemotherapy is less effective on this dying inner part of the
tumor.
DR HENDERSON: It is an irony because intuitively you would think that
bevacizumab would have exactly the opposite effect. In other words, we have
always known that necrosis is one of the problems with delivering chemotherapy
to the inside of the tumor. Tumors don’t have a good vascular system,
so there’s not very much oxygen in there. Therefore, radiation therapy isn’t
as effective, and chemotherapy is less effective on this dying inner part of the
tumor.
So you would think that an anti-angiogenesis agent, which kills these blood vessels, would be antagonistic rather than synergistic with chemotherapy. The effects we’re seeing of increased activity of chemotherapy and bevacizumab in colon, breast, lung and renal cancer appear to be good evidence of synergy.
It is now necessary to go back to the laboratory with the understanding that these agents are working differently than hypothesized.
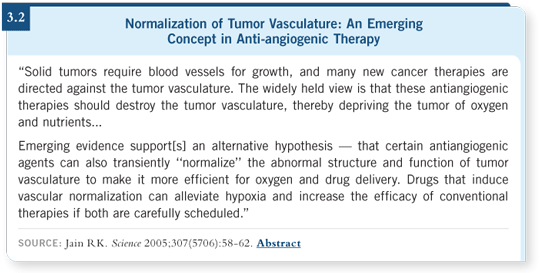
The preclinical data obviously suggest that, in the face of bevacizumab, there is less chaos. In other words, the vasculature is less chaotic and more effective and therefore can deliver more drug (3.2).
Track 11
![]() DR HENDERSON: I see that happening even in my own clinic. I started a
couple of patients in the last few weeks on dose-dense adjuvant chemotherapy
and discussed it with some of my colleagues, and in fact, they are doing this in
the university setting. In CALGB-9741, which compared sequential doxorubicin,
paclitaxel and cyclophosphamide versus concurrent AC followed by
paclitaxel at 14- and 21-day intervals, we can’t separate which is the critical
factor — the AC or the taxane (Hudis 2005). We will have to wait and see
what the science says.
DR HENDERSON: I see that happening even in my own clinic. I started a
couple of patients in the last few weeks on dose-dense adjuvant chemotherapy
and discussed it with some of my colleagues, and in fact, they are doing this in
the university setting. In CALGB-9741, which compared sequential doxorubicin,
paclitaxel and cyclophosphamide versus concurrent AC followed by
paclitaxel at 14- and 21-day intervals, we can’t separate which is the critical
factor — the AC or the taxane (Hudis 2005). We will have to wait and see
what the science says.

Editor’s Note:
Reality check
Interviews
Marc E Lippman, MD
- Select publications
C Kent Osborne, MD
- Select publications
I Craig Henderson, MD
- Select publications
Charles E Geyer Jr, MD
- Select publications
Frank A Vicini, MD
- Select publications
Miami Beach Cancer Conference Tumor Panel Discussion on Systemic Therapy of Metastatic Disease
- Select publications