
 |
||||||||

| Tracks 1-13 | ||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 4-6
![]() DR LOVE: Can you discuss the 100-month follow-up data from the
ATAC trial?
DR LOVE: Can you discuss the 100-month follow-up data from the
ATAC trial?
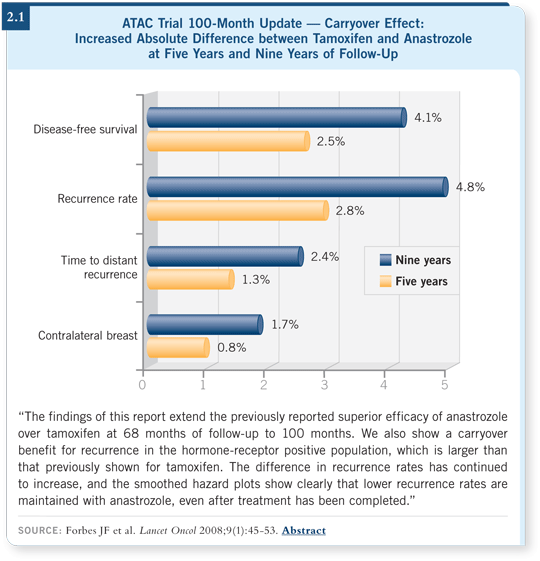
![]() PROF FORBES: With this new data set, we learned some important concepts
for practice. First, the advantage of anastrozole over tamoxifen is maintained at
least through nine years. It has a carryover effect — the benefit continues after
the treatment is stopped. In particular for the post-treatment period from years
five to nine, the advantage of anastrozole over tamoxifen remains significant.
Furthermore, the absolute difference in the rate of breast cancer relapse increases
in magnitude from 2.8 percent at five years to 4.8 percent at nine years (Forbes
2008; [2.1]).
PROF FORBES: With this new data set, we learned some important concepts
for practice. First, the advantage of anastrozole over tamoxifen is maintained at
least through nine years. It has a carryover effect — the benefit continues after
the treatment is stopped. In particular for the post-treatment period from years
five to nine, the advantage of anastrozole over tamoxifen remains significant.
Furthermore, the absolute difference in the rate of breast cancer relapse increases
in magnitude from 2.8 percent at five years to 4.8 percent at nine years (Forbes
2008; [2.1]).
A somewhat unexpected and especially pleasing finding was that upon completion of the treatment, no difference was apparent in the risk of fracture between tamoxifen and anastrozole (2. 2). The other surprising finding was the much lower rate of endometrial cancer in women receiving anastrozole compared to those on tamoxifen (Forbes 2008; [2.2]).
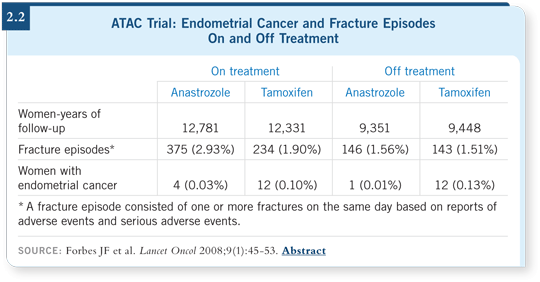
This seems to be consistent with a prevention role, and anastrozole may be a future candidate for primary prevention research in endometrial cancer.
When we design these trials , we define the endpoints up front. Overall survival is a safety and efficacy endpoint, which is important but may not tell you a lot about the signal from breast cancer events.
Overall survival becomes diluted by nonbreast cancer mortality. The most indicative endpoint is time to recurrence, which is a measure of breast cancer events — metastatic, local or contralateral.
The ATAC trial produced clear evidence that anastrozole afforded a reduction in total breast cancer events in addition to contralateral breast cancer events. In this trial, we also evaluated time to distant recurrences, which are metastatic events, and they were also significantly reduced.
![]() DR LOVE: What was the magnitude of the reduction in contralateral breast
cancer with anastrozole as compared to tamoxifen?
DR LOVE: What was the magnitude of the reduction in contralateral breast
cancer with anastrozole as compared to tamoxifen?
![]() PROF FORBES: The risk was reduced from 1.8 percent to 1.0 percent at five
years. The absolute reduction had increased by the nine-year point, the risk
being reduced from 4.2 percent to 2.5 percent (Forbes 2008; [2.1]).
PROF FORBES: The risk was reduced from 1.8 percent to 1.0 percent at five
years. The absolute reduction had increased by the nine-year point, the risk
being reduced from 4.2 percent to 2.5 percent (Forbes 2008; [2.1]).
If the five-year contralateral breast cancer rate for patients on tamoxifen is 1.8 percent, that’s about 0.3 percent a year or about three in a thousand, which is approximately the same risk as that of a 60-year-old woman in the US population developing new breast cancer. That rate is close to halved with anastrozole — it’s a 40 percent reduction in relative terms.
![]() DR LOVE: What would you expect you might see for an aromatase inhibitor
versus placebo in terms of the reduction in second-primary breast cancer?
DR LOVE: What would you expect you might see for an aromatase inhibitor
versus placebo in terms of the reduction in second-primary breast cancer?
![]() PROF FORBES: You can estimate about a 75 percent reduction. It’s close to
half with tamoxifen and another quarter with anastrozole relative to tamoxifen.
If you had a primary prevention trial using anastrozole, you might
prevent approximately 75 percent of the hormone-sensitive breast cancer cases,
which would be a major impact.
PROF FORBES: You can estimate about a 75 percent reduction. It’s close to
half with tamoxifen and another quarter with anastrozole relative to tamoxifen.
If you had a primary prevention trial using anastrozole, you might
prevent approximately 75 percent of the hormone-sensitive breast cancer cases,
which would be a major impact.
![]() DR LOVE: What about overall survival?
DR LOVE: What about overall survival?
![]() PROF FORBES: Among the patients receiving anastrozole, overall mortality
is reduced by three percent, which is not statistically significant. Breast cancer
mortality is reduced by 10 percent, and that is not conventionally significant
either (Forbes 2008).
PROF FORBES: Among the patients receiving anastrozole, overall mortality
is reduced by three percent, which is not statistically significant. Breast cancer
mortality is reduced by 10 percent, and that is not conventionally significant
either (Forbes 2008).
The significance, of course, depends on the number of events that are occurring and the magnitude of the difference, and either of these can show up as a particularly small p-value.
Conventionally, we regard overall survival as the gold standard of treatment effect, but we treat many chronic illnesses, such as osteoarthritis and rheumatoid arthritis, without taking survival into consideration. We won’t say that we will not treat because there is no demonstrated overall survival benefit.
Tracks 11, 13
![]() DR LOVE: Let’s talk more about the data on bone fractures that you
reported from ATAC.
DR LOVE: Let’s talk more about the data on bone fractures that you
reported from ATAC.
![]() PROF FORBES: The data on bone fractures from ATAC are informative and
pleasantly surprising. For a number of years, we’ve been aware of the increased
risk of fractures associated with the aromatase inhibitors compared to tamoxifen.
PROF FORBES: The data on bone fractures from ATAC are informative and
pleasantly surprising. For a number of years, we’ve been aware of the increased
risk of fractures associated with the aromatase inhibitors compared to tamoxifen.
What was surprising was that upon completion of the treatment, no difference was detectable in the risk of fractures with anastrozole compared to tamoxifen (Forbes 2008; [2.2]).
It is interesting that no detrimental carryover effect is evident here. Almost as soon as you stop the treatment — within one year — the difference is gone.
I believe we need to be a little cautious about leaping to safety reassurance at this point, however, because the types of fracture risk may vary: Hip fractures may well be different from vertebral fractures.
These are different types of bone, and I believe we need much longer follow-up to be sure that there isn’t some unsuspected, longer-term effect on hip fractures.
![]() DR LOVE: Can you talk about the bone substudy from ATAC that had previously
been reported (Eastell 2008), and specifically the question of what
happens to the women who start out with normal bone density?
DR LOVE: Can you talk about the bone substudy from ATAC that had previously
been reported (Eastell 2008), and specifically the question of what
happens to the women who start out with normal bone density?
![]() PROF FORBES: The bone substudy in ATAC was designed to evaluate the
effect of anastrozole on bone density and potential longer-term strategies to
correct it. We learned that women who started out with a normal bone density
may develop osteopenia but will not develop osteoporosis (Eastell 2008).
PROF FORBES: The bone substudy in ATAC was designed to evaluate the
effect of anastrozole on bone density and potential longer-term strategies to
correct it. We learned that women who started out with a normal bone density
may develop osteopenia but will not develop osteoporosis (Eastell 2008).
Track 9
![]() DR LOVE: Would you discuss the rationale for the LATER trial?
DR LOVE: Would you discuss the rationale for the LATER trial?
![]() PROF FORBES: The LATER study is a double-blind, randomized trial that
compares letrozole to placebo (2.3). It’s the only placebo-controlled prevention
trial that’s being conducted in this context, so it is potentially a paradigm-shifting
study.
PROF FORBES: The LATER study is a double-blind, randomized trial that
compares letrozole to placebo (2.3). It’s the only placebo-controlled prevention
trial that’s being conducted in this context, so it is potentially a paradigm-shifting
study.
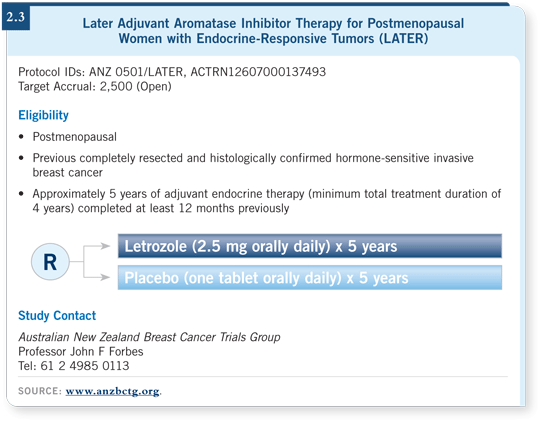
The trial addresses the issue of the ongoing, long-term risk of relapse for postmenopausal patients who completed primary treatment for an endocrine sensitive breast tumor, such as five years of adjuvant tamoxifen, at least one year ago.
The ongoing risk of relapse for these women is approximately two percent per year. Interestingly, this group of patients has not received attention as a high-risk population.
By way of comparison, the eligibility criteria for the primary prevention trials required a five-year risk of 1.7 percent. Women in these trials were considered to be at high risk, with a risk of relapse of only 0.3 percent per year. The postmenopausal patients that I’m talking about have a five-to tenfold greater risk than those in the prevention trials.
Another example to consider is the annual risk of breast cancer for women with BRCA mutations. At worst, a 35-year-old woman with this mutation and a 40-year life expectancy has an 80 percent chance of developing breast cancer, which is a risk of two percent per year during 40 years. It’s the same risk for the population I’m discussing, women at notably high risk who have been neglected.
Our strategy for managing their risk has been surveillance. We need to approach risk management much more broadly than simply performing a mammogram and noting the family history on a single occasion.
We know tamoxifen prevents contralateral breast cancer and that aromatase inhibitors are approximately twice as good as tamoxifen at preventing these tumors.
It is likely that an aromatase inhibitor some years after diagnosis and completion of treatment would be a positive step. We could probably prevent half the relapses in these women.
I view the LATER study as a primary prevention trial as much as a therapeutic trial because the biology behind these late relapses is unclear. Some of them may be new tumor growth.
Some of them may be dormant tumors that have been there a long time from the time of the original diagnosis, but dormancy is a vague concept.
EDITOR'S NOTE
Bev, BETH, BEATRICE and the next big moment in oncology
research: NSABP-C-08
Neil Love, MD
- Select publication
INTERVIEWS
Daniel F Hayes, MD
- Select publications
John F Forbes, MD
- Select publications
Julie R Gralow, MD
- Select publications
John F R Robertson,
MB, ChB, BSc, MD
- Select publications
Roundtable Discussion
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity