
 |
||||||||

| Tracks 1-12 | ||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-2
![]() DR LOVE: Could you discuss the results from the NEWEST trial that
were presented at the 2007 San Antonio Breast Cancer Symposium?
DR LOVE: Could you discuss the results from the NEWEST trial that
were presented at the 2007 San Antonio Breast Cancer Symposium?
![]() PROF ROBERTSON: This was a neoadjuvant, Phase II, open-label study
for postmenopausal women with estrogen-sensitive tumors. Patients were
randomly assigned to either standard-dose or high-dose fulvestrant. The
patients assigned to the high-dose group received fulvestrant at 500 milligrams
per month, with 500 milligrams on day 14 of the first month to reach a
plateau more quickly. The standard dose of fulvestrant was 250 milligrams per
month (Kuter 2007).
PROF ROBERTSON: This was a neoadjuvant, Phase II, open-label study
for postmenopausal women with estrogen-sensitive tumors. Patients were
randomly assigned to either standard-dose or high-dose fulvestrant. The
patients assigned to the high-dose group received fulvestrant at 500 milligrams
per month, with 500 milligrams on day 14 of the first month to reach a
plateau more quickly. The standard dose of fulvestrant was 250 milligrams per
month (Kuter 2007).
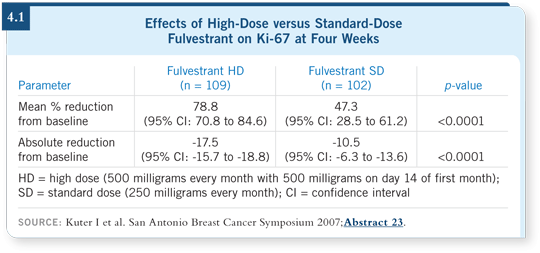
This trial enrolled patients with tumors larger than two centimeters. Ultrasound examinations and tumor biopsies were performed at baseline, week four and at the time of surgery. In addition to the primary endpoint measurement of Ki-67, other markers of hormone sensitivity, such as ER and PR, were measured. The results indicate that the 500-mg dose caused significantly greater downregulation of the proliferation marker Ki-67 at four weeks than the 250-mg dose (Kuter 2007; [4.1]).
![]() DR LOVE: Can you provide a background of prior related neoadjuvant studies?
DR LOVE: Can you provide a background of prior related neoadjuvant studies?
![]() PROF ROBERTSON: One was a study published in Cancer Research in which we
evaluated fulvestrant at 50, 125 and 250 milligrams (Robertson 2001). Results
indicated a dose-dependent downregulation of ER, Ki-67 and PR. Data pointed
toward antiestrogens being antiproliferative agents, the implication being that
increased downregulation of Ki-67 results in increased fulvestrant efficacy.
PROF ROBERTSON: One was a study published in Cancer Research in which we
evaluated fulvestrant at 50, 125 and 250 milligrams (Robertson 2001). Results
indicated a dose-dependent downregulation of ER, Ki-67 and PR. Data pointed
toward antiestrogens being antiproliferative agents, the implication being that
increased downregulation of Ki-67 results in increased fulvestrant efficacy.
The second piece of information comes from a Phase III trial in which we evaluated fulvestrant versus anastrozole (Howell 2005). We compared fulvestrant at 250 milligrams and 125 milligrams to anastrozole. People often don’t remember that this was initially a three-arm trial. The 125-mg dose was stopped due to lack of disease response. In fact, when we plotted the time to progression, it was significantly shorter than with the 250-mg dose.
Those two pieces of information together implied that this dose-dependent downregulation of ER was clinically important and not just an epiphenomenon. I was also the principal investigator of a study of fulvestrant at 250 milligrams in premenopausal patients, which demonstrated no effect on ER or Ki-67 (Robertson 2007a).
A subsequent study by Mike Dixon demonstrated that in premenopausal women, fulvestrant at 750 milligrams caused a similar downregulation of ER and effect on Ki-67 to what 250 milligrams of fulvestrant did in postmenopausal women (Young 2008). It all began to appear as though the downregulation of ER was important, and that was linked to proliferation.
In the first study evaluating preoperative fulvestrant, a short-acting formulation was administered as a daily subcutaneous injection of either six or 18 milligrams (DeFriend 1994). Results showed greater downregulation of ER and greater control of Ki-67 compared to long-acting fulvestrant. So the question was, by increasing the dose of fulvestrant beyond 250 milligrams, could we produce this increased downregulation?
Tracks 4-5
![]() DR LOVE: Do you think it’s reasonable to consider ovarian suppression in
combination with fulvestrant for a premenopausal patient with metastatic
breast cancer?
DR LOVE: Do you think it’s reasonable to consider ovarian suppression in
combination with fulvestrant for a premenopausal patient with metastatic
breast cancer?
![]() PROF ROBERTSON: Yes, I believe it is. Gunter Steger’s work shows response
rates in the range that one would expect from an effective endocrine agent
(Steger 2005). I believe that if you’ve used other, perhaps more established,
options, such as goserelin and tamoxifen or goserelin and anastrozole, and
you’re searching for an alternative, then that’s reasonable for a hormone-sensitive
patient.
PROF ROBERTSON: Yes, I believe it is. Gunter Steger’s work shows response
rates in the range that one would expect from an effective endocrine agent
(Steger 2005). I believe that if you’ve used other, perhaps more established,
options, such as goserelin and tamoxifen or goserelin and anastrozole, and
you’re searching for an alternative, then that’s reasonable for a hormone-sensitive
patient.
![]() DR LOVE: What current clinical trials are evaluating fulvestrant?
DR LOVE: What current clinical trials are evaluating fulvestrant?
![]() PROF ROBERTSON: The FACT study is evaluating the clinical responses
to anastrozole monotherapy versus anastrozole/fulvestrant. That will be an
important contribution to ascertaining whether we should be combining these
drugs.
PROF ROBERTSON: The FACT study is evaluating the clinical responses
to anastrozole monotherapy versus anastrozole/fulvestrant. That will be an
important contribution to ascertaining whether we should be combining these
drugs.
A second important study is one we are running in the United Kingdom, whereby in a presurgical setting, we’re administering fulvestrant at 500 milligrams, fulvestrant at 500 milligrams with an aromatase inhibitor or the aromatase inhibitor alone.
Tracks 6-7
![]() DR LOVE: Can you envision fulvestrant being integrated into the adjuvant
setting in any way other than combining it with an aromatase inhibitor?
DR LOVE: Can you envision fulvestrant being integrated into the adjuvant
setting in any way other than combining it with an aromatase inhibitor?
![]() PROF ROBERTSON: I believe we might want to integrate fulvestrant with
certain signal-transduction or growth factor inhibitors. We recently reported
that fulvestrant had a 40 percent clinical benefit rate among postmenopausal
women with HER2-positive tumors (Robertson 2007b).
PROF ROBERTSON: I believe we might want to integrate fulvestrant with
certain signal-transduction or growth factor inhibitors. We recently reported
that fulvestrant had a 40 percent clinical benefit rate among postmenopausal
women with HER2-positive tumors (Robertson 2007b).
These patients were receiving anything from second-to fifth-line therapy. That’s good activity for that group of patients with previously treated, HER2-positive disease.
![]() DR LOVE: What about fulvestrant for patients with HER2-positive breast
cancer?
DR LOVE: What about fulvestrant for patients with HER2-positive breast
cancer?
![]() PROF ROBERTSON: I believe tamoxifen doesn’t work as well in patients with
HER2-positive tumors, but it appears as though fulvestrant is effective. In
that group of patients, fulvestrant was effective whether or not patients had
received or responded to trastuzumab (Robertson 2007b).
PROF ROBERTSON: I believe tamoxifen doesn’t work as well in patients with
HER2-positive tumors, but it appears as though fulvestrant is effective. In
that group of patients, fulvestrant was effective whether or not patients had
received or responded to trastuzumab (Robertson 2007b).
I believe that combining fulvestrant with an aromatase inhibitor is not the only alternative. In advanced HER2-positive disease, trastuzumab monotherapy induces only a 30 percent response rate (Vogel 2002). Perhaps the combination of fulvestrant and trastuzumab might increase the response rates.
![]() DR LOVE: What are your thoughts about the TAnDEM trial?
DR LOVE: What are your thoughts about the TAnDEM trial?
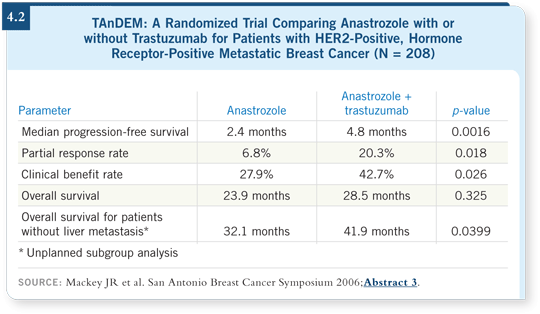
![]() PROF ROBERTSON: The results from the TAnDEM trial demonstrate an
increase in response rate and time to progression but no difference in overall
survival with the combination of anastrozole and trastuzumab (Mackey 2006;
[4.2]). I believe that raises the question as to whether or not sequential therapy
is as effective as the combination.
PROF ROBERTSON: The results from the TAnDEM trial demonstrate an
increase in response rate and time to progression but no difference in overall
survival with the combination of anastrozole and trastuzumab (Mackey 2006;
[4.2]). I believe that raises the question as to whether or not sequential therapy
is as effective as the combination.
Track 8
![]() DR LOVE: What do you think about the LATER trial evaluating delayed
aromatase inhibitor therapy, started one year or more after a patient
completes adjuvant endocrine therapy?
DR LOVE: What do you think about the LATER trial evaluating delayed
aromatase inhibitor therapy, started one year or more after a patient
completes adjuvant endocrine therapy?
![]() PROF ROBERTSON: That trial randomly assigns postmenopausal patients
who completed adjuvant endocrine therapy at least one year ago — so they’re
between six and 20 years from their initial treatment — to letrozole versus
placebo. You have a gap between therapies, which makes this an interesting
study because essentially it asks, do we need continuous therapy to be able to
intervene in breast cancer?
PROF ROBERTSON: That trial randomly assigns postmenopausal patients
who completed adjuvant endocrine therapy at least one year ago — so they’re
between six and 20 years from their initial treatment — to letrozole versus
placebo. You have a gap between therapies, which makes this an interesting
study because essentially it asks, do we need continuous therapy to be able to
intervene in breast cancer?
I believe that data from previous trials suggest we don’t. When tamoxifen first became adjuvant therapy, a French study evaluated patients more than two years after surgery who’d had 2-cm tumors — a reasonable risk of recurrence. It randomly assigned patients to tamoxifen or placebo and showed a significant benefit in favor of tamoxifen, so I believe it showed that we can intervene later (Delozier 2000).
The MA17 study also demonstrates this in that patients who were on the placebo control arm were allowed to take letrozole when the study was closed. This wasn’t randomized, but those patients also have a reduction in the risk of recurrence compared to those who did not begin letrozole (Robert 2006).
The third, provocative trial that supports this is the HERA trial. Patients were randomly assigned to no trastuzumab versus one or two years of trastuzumab.
Again, after the first analysis, patients who didn’t receive trastuzumab were allowed to receive it as delayed therapy. And again, not randomized, this study also reported a lower recurrence rate in the group who received delayed trastuzumab compared to those who did not.
I believe the data from all these trials show that delayed treatment can still reduce a patient’s long-term risk of recurrence.
EDITOR'S NOTE
Bev, BETH, BEATRICE and the next big moment in oncology
research: NSABP-C-08
Neil Love, MD
- Select publication
INTERVIEWS
Daniel F Hayes, MD
- Select publications
John F Forbes, MD
- Select publications
Julie R Gralow, MD
- Select publications
John F R Robertson,
MB, ChB, BSc, MD
- Select publications
Roundtable Discussion
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity