
 |
||||||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 6
![]() DR LOVE: Can you discuss the results from your paper in The New
England Journal of Medicine evaluating the efficacy of taxanes based on
HER2 status?
DR LOVE: Can you discuss the results from your paper in The New
England Journal of Medicine evaluating the efficacy of taxanes based on
HER2 status?
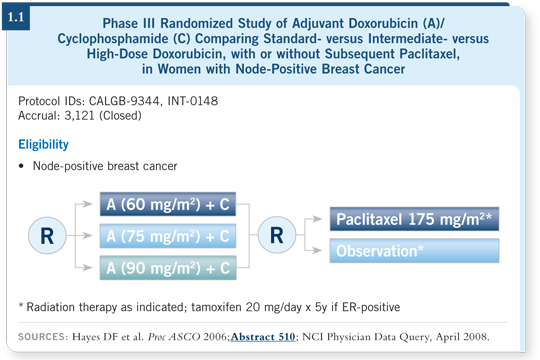
![]() DR HAYES: I had the opportunity to work with the CALGB to prospectively
collect tissue blocks from the patients enrolled in CALGB-9344, which evaluated AC with or without paclitaxel (1.1). We proposed that we might find a
benefit with higher doses of doxorubicin for patients with HER2-positive
disease. We didn’t know what to propose for paclitaxel. Preclinical data
suggested that HER2-positive cells were resistant to paclitaxel and docetaxel.
That was one hypothesis, but at the time we wrote the protocol, some clinical
data suggested the opposite.
DR HAYES: I had the opportunity to work with the CALGB to prospectively
collect tissue blocks from the patients enrolled in CALGB-9344, which evaluated AC with or without paclitaxel (1.1). We proposed that we might find a
benefit with higher doses of doxorubicin for patients with HER2-positive
disease. We didn’t know what to propose for paclitaxel. Preclinical data
suggested that HER2-positive cells were resistant to paclitaxel and docetaxel.
That was one hypothesis, but at the time we wrote the protocol, some clinical
data suggested the opposite.
![]() DR LOVE: This study began in 1994 when HER2 assays were not being done.
How did you obtain the data?
DR LOVE: This study began in 1994 when HER2 assays were not being done.
How did you obtain the data?
![]() DR HAYES: Once CALGB-9344 was completed, we wrote a separate protocol
to evaluate HER2 with three different assays and incorporate the ER data
from the primary sites. We conducted the HER2 testing in investigational
laboratories. Ann Thor performed HER2 analysis with CB11 staining. Don
Weaver used the HercepTest™ at the University of Vermont, and Lynn Dressler
performed the PathVysion® FISH analysis at the University of North Carolina.
We did not retest the ER status, but we’re doing that now.
DR HAYES: Once CALGB-9344 was completed, we wrote a separate protocol
to evaluate HER2 with three different assays and incorporate the ER data
from the primary sites. We conducted the HER2 testing in investigational
laboratories. Ann Thor performed HER2 analysis with CB11 staining. Don
Weaver used the HercepTest™ at the University of Vermont, and Lynn Dressler
performed the PathVysion® FISH analysis at the University of North Carolina.
We did not retest the ER status, but we’re doing that now.
Our first finding was that regardless of HER2 status, no consistent benefit was evident from higher doses of doxorubicin (Hayes 2007). The relative benefits of doxorubicin reach a plateau at about 60 mg/m2. It doesn’t matter if your disease is HER2-negative or HER2-positive — going above 60 mg/m2 doesn’t appear to bring additional benefit. It’s bad news because I’d like to make progress, but it’s good news because you can have a lot of toxicity at those higher levels of doxorubicin.
Regardless of which of the three assays we used, we determined that a substantial benefit occurred with the addition of paclitaxel for patients with HER2-positive disease, irrespective of ER status. If the patients had HER2-positive disease, ER-negative or ER-positive, they benefited from paclitaxel. Patients with HER2-negative disease benefited less.
We then put the findings together. Statistically it is complicated because now we have a three-way interaction: ER-positive or ER-negative, HER2-positive or HER2-negative and paclitaxel or no paclitaxel. A statistically significant result was recorded for interaction between HER2 and paclitaxel, but we haven’t tried to assess the ER-HER2-paclitaxel interaction.
We found that regardless of HER2 status, patients with ER-negative disease benefited from the addition of paclitaxel, and regardless of ER status, patients with HER2-positive disease benefited. But when we evaluated the subgroup with ER-positive and HER2-negative disease — which accounted for 50 percent of the patients in this clinical trial — the curves for paclitaxel versus no paclitaxel overlapped (Hayes 2007; [1.2]). This suggests that we could avoid the extra four cycles of a modestly toxic and expensive drug, even for patients with node-positive disease.
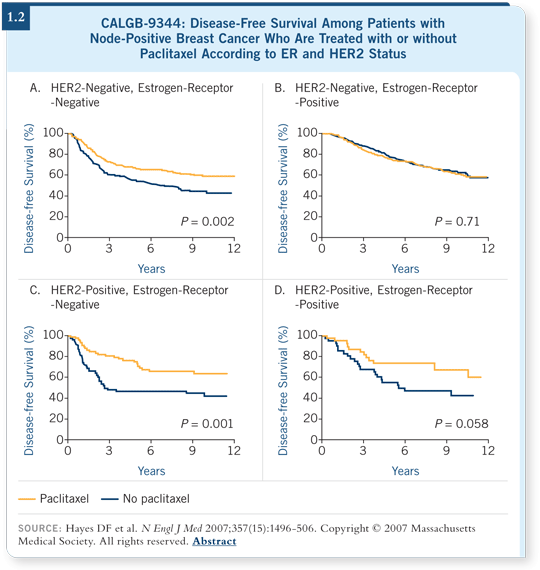
This was, however, a retrospective subset analysis of a single trial. I don’t believe that women with ER-positive, HER2-negative, node-positive disease should have paclitaxel withheld at this point.
We have seen a substantial reduction in mortality as a result of chemotherapy in general for patients with node-positive disease during the past 20 years. We need to be sure that these findings are correct before we move forward.
![]() DR LOVE: Do these results reflect the efficacy of paclitaxel specifically, or is
this more about chemotherapy in general in this subset of patients?
DR LOVE: Do these results reflect the efficacy of paclitaxel specifically, or is
this more about chemotherapy in general in this subset of patients?
![]() DR HAYES: You’re one step ahead of me. I don’t believe this is specific to
paclitaxel. This is probably a generic chemotherapy effect.
DR HAYES: You’re one step ahead of me. I don’t believe this is specific to
paclitaxel. This is probably a generic chemotherapy effect.
We hinted around at this idea in the paper, but it was not included in the discussion. It would have been speculation because we didn’t have a no-treatment control arm. We did say it was consistent with the effects seen by Don Berry (Berry 2006).
Track 9
![]() DR LOVE: Can you comment on the recent SWOG analysis of the utility
of the Oncotype DX assay for women with node-positive breast cancer?
DR LOVE: Can you comment on the recent SWOG analysis of the utility
of the Oncotype DX assay for women with node-positive breast cancer?
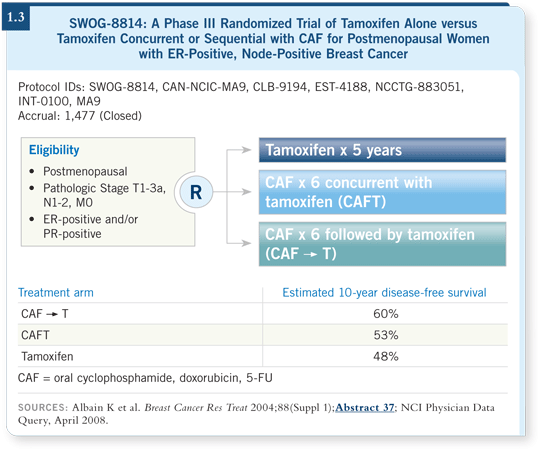
![]() DR HAYES: Kathy Albain led a study (SWOG-8814) for postmenopausal
patients with node-positive, ER-positive disease who were randomly assigned
to tamoxifen alone, tamoxifen concurrent with CAF or CAF followed by
tamoxifen. SWOG-8814 demonstrated that chemotherapy concurrent with
tamoxifen was not as effective as chemotherapy followed by tamoxifen (Albain
2004; [1.3]).
DR HAYES: Kathy Albain led a study (SWOG-8814) for postmenopausal
patients with node-positive, ER-positive disease who were randomly assigned
to tamoxifen alone, tamoxifen concurrent with CAF or CAF followed by
tamoxifen. SWOG-8814 demonstrated that chemotherapy concurrent with
tamoxifen was not as effective as chemotherapy followed by tamoxifen (Albain
2004; [1.3]).
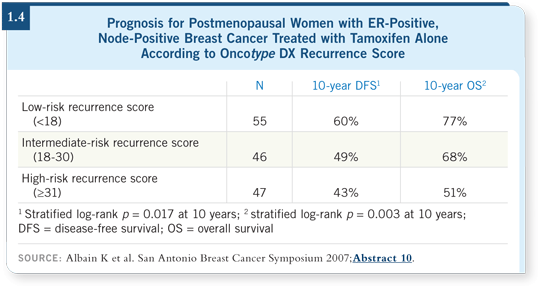
Tissue blocks were available from approximately 40 percent of those patients.
We only compared tamoxifen alone to CAF![]() tamoxifen. To our pleasure, we
saw exactly what we predicted. Among the patients treated with tamoxifen
alone, those with low recurrence scores as determined by Oncotype DX fared
better than those with high recurrence scores (Albain 2007; [1.4]).
tamoxifen. To our pleasure, we
saw exactly what we predicted. Among the patients treated with tamoxifen
alone, those with low recurrence scores as determined by Oncotype DX fared
better than those with high recurrence scores (Albain 2007; [1.4]).
Nodes are still prognostic here. In fact, as many as 40 percent of patients with node-positive disease and low recurrence scores will still experience a recurrence on tamoxifen alone (Albain 2007; [1.4]).
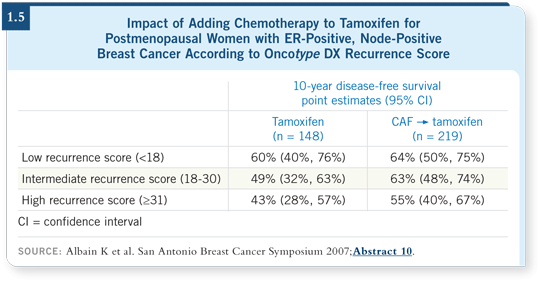
Among the patients with node-positive disease and high recurrence
scores, a statistically significant benefit was seen in disease-free survival with
CAF![]() tamoxifen versus tamoxifen alone. For the patients with low recurrence
scores, the curves were overlapping (Albain 2007; [1.5]). Even in node-positive
disease, chemotherapy may not be effective for patients with high ER,
low HER2 or low Ki-67 — the components of the Oncotype DX assay.
tamoxifen versus tamoxifen alone. For the patients with low recurrence
scores, the curves were overlapping (Albain 2007; [1.5]). Even in node-positive
disease, chemotherapy may not be effective for patients with high ER,
low HER2 or low Ki-67 — the components of the Oncotype DX assay.
![]() DR LOVE: In NSABP-B-20, patients with node-negative disease and high
recurrence scores demonstrated a relative risk reduction of 75 percent with
chemotherapy. What was observed among the patients with node-positive
disease?
DR LOVE: In NSABP-B-20, patients with node-negative disease and high
recurrence scores demonstrated a relative risk reduction of 75 percent with
chemotherapy. What was observed among the patients with node-positive
disease?
![]() DR HAYES: It’s not that large. I believe all of us felt that the B-20 data were
an overestimate. This was a retrospective study with fewer events. I’m not
ready to “pull the trigger” on the node-positive situation on this basis. I
believe we have two provocative retrospective subset analyses that begin to
suggest the same result.
DR HAYES: It’s not that large. I believe all of us felt that the B-20 data were
an overestimate. This was a retrospective study with fewer events. I’m not
ready to “pull the trigger” on the node-positive situation on this basis. I
believe we have two provocative retrospective subset analyses that begin to
suggest the same result.
![]() DR LOVE: What do you mean by “pull the trigger”? What if you have a
patient who has a node-positive tumor and a low recurrence score? Would
it be accurate to say that adjuvant chemotherapy in this situation has an
unproven benefit?
DR LOVE: What do you mean by “pull the trigger”? What if you have a
patient who has a node-positive tumor and a low recurrence score? Would
it be accurate to say that adjuvant chemotherapy in this situation has an
unproven benefit?
![]() DR HAYES: I’d make the opposite argument, which is that for patients with
node-positive disease, adjuvant chemotherapy is of proven benefit regardless
of biological subsets. Almost every guideline recommends chemotherapy for
patients with node-positive disease. The stakes are high in this situation.
DR HAYES: I’d make the opposite argument, which is that for patients with
node-positive disease, adjuvant chemotherapy is of proven benefit regardless
of biological subsets. Almost every guideline recommends chemotherapy for
patients with node-positive disease. The stakes are high in this situation.
I don’t believe nodal status indicates whether chemotherapy will work — biology tells you whether chemotherapy will work. I am not sure whether this is the assay we should use to decide whether to withhold adjuvant chemotherapy from patients with node-positive disease.
The question is whether we should run yet another prospective randomized trial. It would be a large trial because it would be an equivalency trial. Patients with low recurrence scores who received adjuvant tamoxifen would be randomly assigned to modern chemotherapy versus none.
EDITOR'S NOTE
Bev, BETH, BEATRICE and the next big moment in oncology
research: NSABP-C-08
Neil Love, MD
- Select publication
INTERVIEWS
Daniel F Hayes, MD
- Select publications
John F Forbes, MD
- Select publications
Julie R Gralow, MD
- Select publications
John F R Robertson,
MB, ChB, BSc, MD
- Select publications
Roundtable Discussion
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity