
 |
||||||||

| Tracks 1-15 | ||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR LOVE: What is your usual first-line chemotherapy regimen for a
patient with metastatic breast cancer? Do you include bevacizumab?
DR LOVE: What is your usual first-line chemotherapy regimen for a
patient with metastatic breast cancer? Do you include bevacizumab?
![]() DR GRALOW: I believe the data with a taxane/bevacizumab regimen are
strong. For weekly paclitaxel/bevacizumab, a doubling in progression-free survival — in absolute terms, adding six months — is clinically meaningful
(Miller 2007). So the data support using bevacizumab with weekly paclitaxel.
DR GRALOW: I believe the data with a taxane/bevacizumab regimen are
strong. For weekly paclitaxel/bevacizumab, a doubling in progression-free survival — in absolute terms, adding six months — is clinically meaningful
(Miller 2007). So the data support using bevacizumab with weekly paclitaxel.
In the Southwest Oncology Group, we have a lot of experience with the combination of antitubulins and vinca alkaloids — either docetaxel or paclitaxel in combination with vinorelbine.
At ASCO this year, we will be presenting a Phase II study of docetaxel/vinorelbine with trastuzumab for patients with HER2-positive disease.
Docetaxel/vinorelbine is an impressive regimen, whether it’s administered in combination with bevacizumab for HER2-negative disease or with trastuzumab for HER2-positive disease. That’s my experience.
So I tend to use that as an aggressive regimen up front if I want to obtain a rapid response. We have presented data previously on docetaxel/vinorelbine without bevacizumab for patients with HER2-negative disease (Gralow 2005).
Track 3
![]() DR LOVE: Can you review what we know about nab paclitaxel in breast
cancer?
DR LOVE: Can you review what we know about nab paclitaxel in breast
cancer?
![]() DR GRALOW: Nab paclitaxel does not require premedications, has a faster
infusion time and has the ability to deliver somewhat higher doses of the drug.
I believe we are seeing a dose-response effect above what we’ve traditionally
observed with paclitaxel.
DR GRALOW: Nab paclitaxel does not require premedications, has a faster
infusion time and has the ability to deliver somewhat higher doses of the drug.
I believe we are seeing a dose-response effect above what we’ve traditionally
observed with paclitaxel.
Certainly the data with every three-week nab paclitaxel versus paclitaxel are in favor of nab paclitaxel (Gradishar 2005; [5.3]). We have randomized Phase II data showing that when administered weekly, nab paclitaxel may be as good as, if not better than, docetaxel (Gradishar 2007).
It’s a fascinating drug, and I like using it a lot. I like not having to administer steroids and antihistamines and the markedly reduced chance of allergic reactions. I’m excited about trials moving nab paclitaxel into the adjuvant setting.
![]() DR LOVE: What about nab paclitaxel in combination with bevacizumab?
DR LOVE: What about nab paclitaxel in combination with bevacizumab?
![]() DR GRALOW: Absolutely. When I can get insurance company approval, I will
use that regimen.
DR GRALOW: Absolutely. When I can get insurance company approval, I will
use that regimen.
![]() DR LOVE: What do we know about that combination at this point?
DR LOVE: What do we know about that combination at this point?
![]() DR GRALOW: We have no randomized Phase III trial data. However, we do
have safety data. I make the leap that it is the same core drug — paclitaxel
— at a higher dose.
DR GRALOW: We have no randomized Phase III trial data. However, we do
have safety data. I make the leap that it is the same core drug — paclitaxel
— at a higher dose.
I don’t see the need for a randomized Phase III trial to prove efficacy in that setting when I’m substituting a somewhat higher dose of the core drug, paclitaxel.
Track 6
![]() DR LOVE: What about metastatic disease in the patient with a HER2-positive tumor? How would you approach such a case when the patient
has received adjuvant trastuzumab?
DR LOVE: What about metastatic disease in the patient with a HER2-positive tumor? How would you approach such a case when the patient
has received adjuvant trastuzumab?
![]() DR GRALOW: Fortunately, we now have a second HER2-targeted agent with
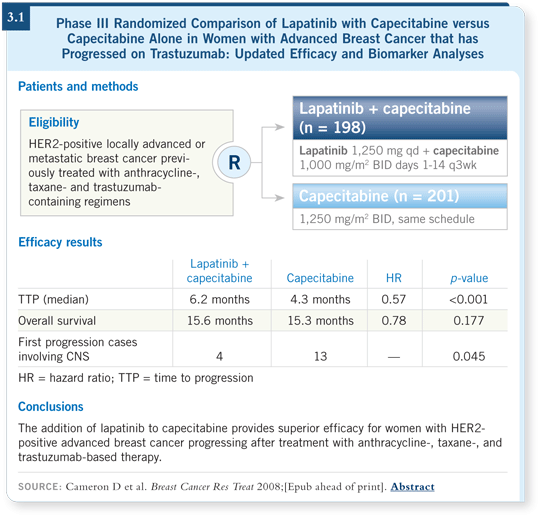
FDA approval. So for patients who progress on or quickly after stopping adjuvant
trastuzumab, I favor using lapatinib, probably in combination with capecitabine
(3.1), depending on what other chemotherapy the patient has received. We have
some data with lapatinib and paclitaxel, in case I wanted to use that drug.
DR GRALOW: Fortunately, we now have a second HER2-targeted agent with
FDA approval. So for patients who progress on or quickly after stopping adjuvant
trastuzumab, I favor using lapatinib, probably in combination with capecitabine
(3.1), depending on what other chemotherapy the patient has received. We have
some data with lapatinib and paclitaxel, in case I wanted to use that drug.
If the interval between stopping adjuvant trastuzumab and the recurrence was long, then I would seriously consider restarting trastuzumab as my first approach.
I’m interested in trials evaluating whether the two drugs in combination are better than either alone, but that is not something I’d do in a clinical setting right now.
We have a couple of other promising HER2-targeted agents in the pipeline. Pertuzumab is a fascinating drug. I’m most fascinated by T-DM1, which is trastuzumab conjugated to a maytansine derivative.
It delivers chemotherapy directly to the tumor cell. I believe that’s smart therapy, with exciting early data (Beeram 2007). Using trastuzumab for drug delivery is a fascinating approach.
Track 7
![]() DR LOVE: Can you describe your experience with lapatinib?
DR LOVE: Can you describe your experience with lapatinib?
![]() DR GRALOW: I’m using it, and I’m certainly seeing beneficial effects. We’re
still in the phase of integrating it into practice. Capecitabine/lapatinib can be
complicated to administer.
DR GRALOW: I’m using it, and I’m certainly seeing beneficial effects. We’re
still in the phase of integrating it into practice. Capecitabine/lapatinib can be
complicated to administer.
It’s a completely oral regimen with many pills. Capecitabine is taken twice a day for only two out of three weeks. Lapatinib is taken once a day. One drug is taken before eating anything, and the other is taken later.
So it’s complicated, and we spend a lot of time teaching, reinforcing and making phone calls to make sure patients haven’t mixed up the drugs. It has involved a learning curve.
The nurses need to know what must be reinforced, and we need to know how to counsel patients better. We have some great teaching materials now. I’ve seen efficacy with lapatinib, but also rash and diarrhea. It’s a regimen we’re still getting comfortable using.
![]() DR LOVE: What have you seen in terms of rash?
DR LOVE: What have you seen in terms of rash?
![]() DR GRALOW: Facial and truncal rash. My group treats only patients with
breast cancer, so we don’t have much experience with EGFR-targeted therapy.
Many practicing oncologists have already learned how to deal with that, but
our group has not had much experience.
DR GRALOW: Facial and truncal rash. My group treats only patients with
breast cancer, so we don’t have much experience with EGFR-targeted therapy.
Many practicing oncologists have already learned how to deal with that, but
our group has not had much experience.
We’ve learned of some topical treatments that we can use. We don’t usually use oral antibiotics, but we have done so. We’re getting better at managing the rash.
From the patients’ standpoint, the rash is visible. They can tolerate it on their chest if they can cover it. When it’s on their face, however, they don’t like to be labeled or have people ask about it.
Track 8
![]() DR LOVE: Can you talk about brain metastases in patients with HER2-positive breast cancer and where lapatinib might fit in?
DR LOVE: Can you talk about brain metastases in patients with HER2-positive breast cancer and where lapatinib might fit in?
![]() DR GRALOW: We’re clearly seeing more brain metastases as first or dominant
sites of recurrence in patients with HER2-positive disease. Is it related to the biology of the HER2 tumor cell? My perspective is that we’re controlling all
the other sites of disease better.
DR GRALOW: We’re clearly seeing more brain metastases as first or dominant
sites of recurrence in patients with HER2-positive disease. Is it related to the biology of the HER2 tumor cell? My perspective is that we’re controlling all
the other sites of disease better.
Patients aren’t dying as quickly of liver disease, and they’re living long enough to manifest the symptoms of their brain metastases, another common site of recurrence.
It’s partly biology and partly that we’re doing better at controlling the disease in the rest of the body.
Trastuzumab is a large monoclonal antibody that shouldn’t be able to cross the intact blood-brain barrier. Some anecdotal case reports, however, indicate that once you have a large metastasis that disrupts the blood-brain barrier, you can obtain tumor shrinkage with trastuzumab.
Lapatinib certainly penetrates the blood-brain barrier, and we have, again, some anecdotal evidence suggesting that we can achieve tumor shrinkage with lapatinib alone. Nancy Lin and the group at Dana Farber have conducted some elegant studies for patients with HER2-positive brain metastases who received radiation therapy and whose disease was progressing. They added lapatinib as a single agent, and then at progression or with no response, they added capecitabine (Lin 2007).
Using conventional measures, lapatinib alone has not produced much of a response. It’s making a dent, but it doesn’t meet the classic response criteria.
When patients on those studies experienced disease progression or no response on lapatinib, the next step was adding capecitabine, and quite a few responses then met conventional criteria (Lin 2007). Whether it was the combination of capecitabine/lapatinib or capecitabine alone, I’m not sure.
I’m also tantalized by evidence in the Phase III capecitabine/lapatinib versus capecitabine-alone trial, which indicated a numerical trend toward fewer brain metastases with lapatinib (Geyer 2006).
Will that translate in the adjuvant setting to a real difference that can improve survival? I don’t know. We will certainly be paying attention to that in the recently opened, international ALTTO trial for patients with HER2-positive disease.
Track 9
![]() DR LOVE: Would you describe the design of the ALTTO trial?
DR LOVE: Would you describe the design of the ALTTO trial?
![]() DR GRALOW: ALTTO is a huge, international undertaking between the
Breast International Group and the North American Breast Intergroup.
DR GRALOW: ALTTO is a huge, international undertaking between the
Breast International Group and the North American Breast Intergroup.
The backbone chemotherapy can vary depending on where you live. In the US, we will predominately use an anthracycline and then weekly paclitaxel, with four different ways of administering the HER2-targeted therapy: trastuzumab alone, lapatinib alone, both agents together or a sequence of the two agents with a washout period (3.2).

Everyone receives one year of the HER2-targeted therapy. We will carefully examine the efficacy and toxicity with respect to the heart.
![]() DR LOVE: It is interesting that one of the arms does not include trastuzumab.
DR LOVE: It is interesting that one of the arms does not include trastuzumab.
![]() DR GRALOW: Much debate goes on about that arm and whether it is ethical.
It’s standard in this country to use trastuzumab in that setting: Are we
omitting an effective therapy in favor of an as-yet unproven therapy? In the
metastatic setting, the data with lapatinib are impressive. I’ve been increasingly
reassured that there’s activity, especially in combination with paclitaxel, as
Angelo Di Leo presented at ASCO 2007 (Di Leo 2007).
DR GRALOW: Much debate goes on about that arm and whether it is ethical.
It’s standard in this country to use trastuzumab in that setting: Are we
omitting an effective therapy in favor of an as-yet unproven therapy? In the
metastatic setting, the data with lapatinib are impressive. I’ve been increasingly
reassured that there’s activity, especially in combination with paclitaxel, as
Angelo Di Leo presented at ASCO 2007 (Di Leo 2007).
![]() DR LOVE: Can you talk about the BETH trial that the NSABP and CIRG
are running?
DR LOVE: Can you talk about the BETH trial that the NSABP and CIRG
are running?
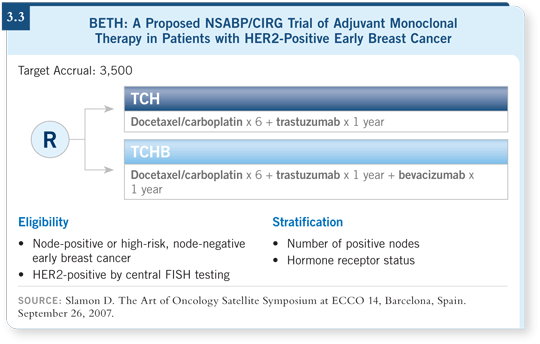
![]() DR GRALOW: Another important issue is the addition of bevacizumab to chemotherapy
and trastuzumab. In the metastatic setting, data for trastuzumab with
bevacizumab presented by Mark Pegram and others have shown an impressive response rate for those two agents together (Pegram 2006). Some concern
has arisen about cardiac toxicity with trastuzumab and bevacizumab without
chemotherapy, and this will be monitored carefully in the BETH trial (3.3).
DR GRALOW: Another important issue is the addition of bevacizumab to chemotherapy
and trastuzumab. In the metastatic setting, data for trastuzumab with
bevacizumab presented by Mark Pegram and others have shown an impressive response rate for those two agents together (Pegram 2006). Some concern
has arisen about cardiac toxicity with trastuzumab and bevacizumab without
chemotherapy, and this will be monitored carefully in the BETH trial (3.3).
EDITOR'S NOTE
Bev, BETH, BEATRICE and the next big moment in oncology
research: NSABP-C-08
Neil Love, MD
- Select publication
INTERVIEWS
Daniel F Hayes, MD
- Select publications
John F Forbes, MD
- Select publications
Julie R Gralow, MD
- Select publications
John F R Robertson,
MB, ChB, BSc, MD
- Select publications
Roundtable Discussion
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity