
 |
||||||||

| Tracks 1-22 | ||||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Discussion
Tracks 3-10
![]() DR LOVE: Edith, this is a challenging situation in which you need to
achieve a tumor response because the patient is very ill. How would you
be thinking through this case?
DR LOVE: Edith, this is a challenging situation in which you need to
achieve a tumor response because the patient is very ill. How would you
be thinking through this case?
![]() DR PEREZ: We’re seeing these patients with triple-negative tumors who
appear to be faring poorly with standard therapies. The types of agents that
have become interesting to consider in this setting are those that target EGFR
or HER1.
DR PEREZ: We’re seeing these patients with triple-negative tumors who
appear to be faring poorly with standard therapies. The types of agents that
have become interesting to consider in this setting are those that target EGFR
or HER1.
Several chemotherapy drugs appear to have some interesting response rates in the subset of patients with triple-negative disease, although these are based on fairly small numbers of patients.
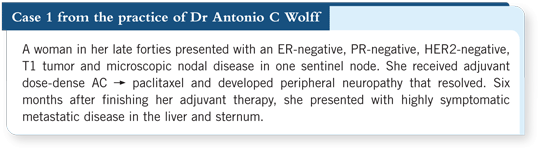
In the past, capecitabine would have been the only FDA-approved agent to administer after disease progression on an anthracycline and a taxane. Now we have FDA approval of ixabepilone in combination with capecitabine for situations such as this one (5.1).
If the patient’s liver enzymes were not too elevated, ixabepilone/capecitabine would be a consideration. If the liver enzymes were elevated, then she would not be eligible for ixabepilone because that’s a contraindication.
Another ongoing study for patients with triple-negative disease involves dasatinib, which is currently used for the treatment of patients with chronic myelogenous leukemia. We are enrolling patients on that study right now.
![]() DR LOVE: Nick, how would you be thinking this through?
DR LOVE: Nick, how would you be thinking this through?
![]() DR ROBERT: We are all concerned about this woman having refractory breast
cancer. You want to be able to provide something that will control her disease,
but you know that whatever you do, it probably will not provide long-term
benefit. If this woman had less aggressive disease, less tumor burden, I would
have considered single-agent capecitabine.
DR ROBERT: We are all concerned about this woman having refractory breast
cancer. You want to be able to provide something that will control her disease,
but you know that whatever you do, it probably will not provide long-term
benefit. If this woman had less aggressive disease, less tumor burden, I would
have considered single-agent capecitabine.
Edith described a large randomized trial that fits this patient to a tee as someone who’s had prior exposure to an anthracycline and a taxane. In that trial, early on, when careful attention was not paid to the liver function tests, they ran into fatal toxicities. It’s important that she have adequate liver function to be exposed to ixabepilone and capecitabine.
That combination is superior to capecitabine in terms of response and progression-free survival (PFS). It was not a great difference in PFS, about 1.5 months, but it achieved a significant p-value (Thomas 2007; [5.1]).
![]() DR LOVE: Antonio, can you follow up on how you treated this patient?
DR LOVE: Antonio, can you follow up on how you treated this patient?
![]() DR WOLFF: This is one example in which I would consider the use of combination
therapy, which I normally do not consider for various reasons. I decided to
do something for which we did not have data. I thought about bevacizumab.
DR WOLFF: This is one example in which I would consider the use of combination
therapy, which I normally do not consider for various reasons. I decided to
do something for which we did not have data. I thought about bevacizumab.
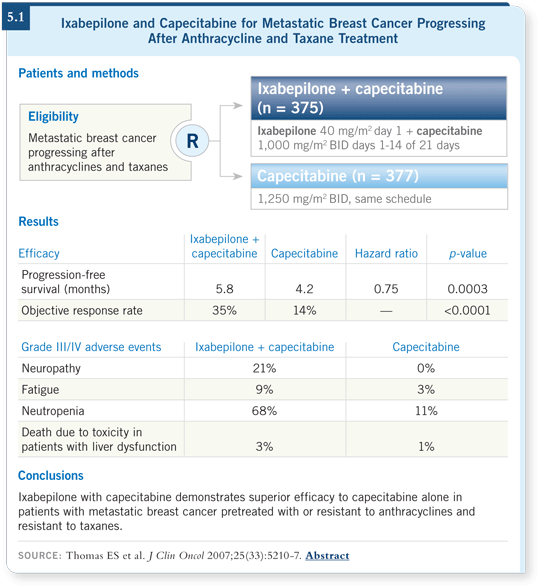
ECOG-E2100, which treated patients with paclitaxel and bevacizumab or paclitaxel alone, allowed prior exposure to paclitaxel if more than 12 months had elapsed (Miller 2007). So this patient would not have been eligible for that study. In my reimbursement environment, however, I would only have the ability to use bevacizumab as first-line therapy for metastatic disease.
![]() DR LOVE: Edith, in that E2100 trial, patients who had received prior
paclitaxel seemed to do at least as well as the other patients.
DR LOVE: Edith, in that E2100 trial, patients who had received prior
paclitaxel seemed to do at least as well as the other patients.
![]() DR PEREZ: The data were evaluated, and having prior exposure to paclitaxel
was not an adverse prognostic factor for progression-free survival benefit from
weekly paclitaxel in combination with bevacizumab (Miller 2007; [5.2]).
DR PEREZ: The data were evaluated, and having prior exposure to paclitaxel
was not an adverse prognostic factor for progression-free survival benefit from
weekly paclitaxel in combination with bevacizumab (Miller 2007; [5.2]).
![]() DR WOLFF: I was out on a limb. This patient had received paclitaxel every
two weeks, and then her cancer relapsed within six months.
DR WOLFF: I was out on a limb. This patient had received paclitaxel every
two weeks, and then her cancer relapsed within six months.
![]() DR ROBERT: Did you think about using nab paclitaxel?
DR ROBERT: Did you think about using nab paclitaxel?
![]() DR WOLFF: I did not consider using nab paclitaxel for various reasons. One is
that it’s not on our formulary.
DR WOLFF: I did not consider using nab paclitaxel for various reasons. One is
that it’s not on our formulary.
![]() DR LOVE: Would you be considering it, Nick?
DR LOVE: Would you be considering it, Nick?
![]() DR ROBERT: Yes, I would.
DR ROBERT: Yes, I would.
![]() DR LOVE: US Oncology has done a lot of important work with nab paclitaxel.
What’s your observation in terms of the neuropathy?
DR LOVE: US Oncology has done a lot of important work with nab paclitaxel.
What’s your observation in terms of the neuropathy?
![]() DR ROBERT: It occurs and is comparable to the neuropathy associated with
paclitaxel. When you stop the drug, it usually disappears or becomes much
better.
DR ROBERT: It occurs and is comparable to the neuropathy associated with
paclitaxel. When you stop the drug, it usually disappears or becomes much
better.
![]() DR LOVE: When you use bevacizumab, do you combine it with paclitaxel or
nab paclitaxel?
DR LOVE: When you use bevacizumab, do you combine it with paclitaxel or
nab paclitaxel?
![]() DR ROBERT: It depends on the situation. If I can use nab paclitaxel, I will. I
believe it’s a better drug.
DR ROBERT: It depends on the situation. If I can use nab paclitaxel, I will. I
believe it’s a better drug.
![]() DR LOVE: Edith, do you believe nab paclitaxel is more efficacious than
paclitaxel?
DR LOVE: Edith, do you believe nab paclitaxel is more efficacious than
paclitaxel?
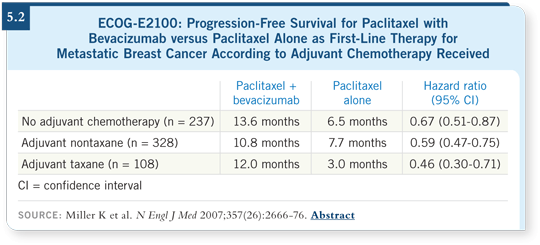
![]() DR PEREZ: We have only one randomized trial, comparing nab paclitaxel
once every three weeks to paclitaxel once every three weeks, and nab paclitaxel
was better (Gradishar 2005; [5.3]). The challenge is that almost nobody
uses paclitaxel once every three weeks. So that comparison is not applicable in
today’s practice.
DR PEREZ: We have only one randomized trial, comparing nab paclitaxel
once every three weeks to paclitaxel once every three weeks, and nab paclitaxel
was better (Gradishar 2005; [5.3]). The challenge is that almost nobody
uses paclitaxel once every three weeks. So that comparison is not applicable in
today’s practice.
We’ve been interested in weekly nab paclitaxel — for example, the work by Joanne Blum evaluating 125 mg/m2 and 100 mg/m2 (Blum 2007). At NCCTG, we conducted a Phase II study of weekly nab paclitaxel in combination with gemcitabine. We saw good activity, although nothing earthshaking (Roy 2007). We will follow this NCCTG trial with another Phase II trial that will use those two chemotherapy drugs in combination with bevacizumab.
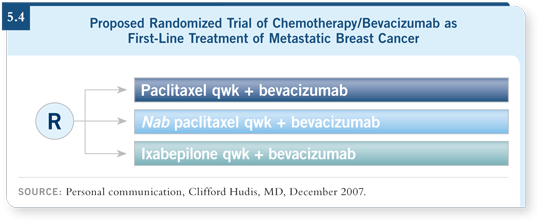
A planned study to be co-led by the CALGB and NCCTG will enroll patients who are eligible to receive first-line chemotherapy for metastatic breast cancer.
Every patient will receive bevacizumab and will then be randomly assigned to weekly paclitaxel, weekly nab paclitaxel or weekly ixabepilone (5.4).
![]() DR LOVE: Edith, what ongoing trials will provide useful information on
bevacizumab in metastatic disease?
DR LOVE: Edith, what ongoing trials will provide useful information on
bevacizumab in metastatic disease?
![]() DR PEREZ: RIBBON 2 (5.5) will help us because 650 patients who have
recently received chemotherapy will be randomly assigned to chemotherapy or
chemotherapy/bevacizumab as second-line therapy.
DR PEREZ: RIBBON 2 (5.5) will help us because 650 patients who have
recently received chemotherapy will be randomly assigned to chemotherapy or
chemotherapy/bevacizumab as second-line therapy.
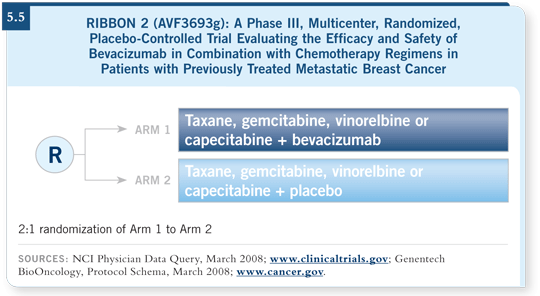
We have other pending studies of bevacizumab in the first-line setting, which we’re eager to learn about when the data are mature. One is the AVADO study, which is evaluating docetaxel versus docetaxel in combination with bevacizumab at two doses.
The RIBBON 1 study is evaluating bevacizumab in combination with a variety of chemotherapy drugs in the first-line setting. This study includes three strata of chemotherapy, which are a taxane, an anthracycline regimen and capecitabine.
![]() DR ROBERT: Interestingly, nab paclitaxel is an option in both the RIBBON 1
and RIBBON 2 trials.
DR ROBERT: Interestingly, nab paclitaxel is an option in both the RIBBON 1
and RIBBON 2 trials.
![]() DR LOVE: Antonio, what happened with your patient?
DR LOVE: Antonio, what happened with your patient?
![]() DR WOLFF: I felt that I was conducting a one-patient clinical trial because I
had no data to support it, but I wanted to provide her with a chance to receive
bevacizumab. She came in and received cycle one of paclitaxel and bevacizumab.
DR WOLFF: I felt that I was conducting a one-patient clinical trial because I
had no data to support it, but I wanted to provide her with a chance to receive
bevacizumab. She came in and received cycle one of paclitaxel and bevacizumab.
She’d had significant ascites, peripheral edema and difficulty walking. She came back one week later, and she was walking, having previously arrived in a wheelchair. Her peripheral edema had disappeared. Her ascites were almost completely gone, and her liver had shrunk after one dose of paclitaxel and bevacizumab.
I had never seen that kind of response before. One could argue that perhaps weekly paclitaxel would have done it, or one could be excited about the possibility that it was the combination of bevacizumab and paclitaxel.
Tracks 14-15

![]() DR LOVE: Antonio, what’s your usual approach for a patient who has
been treated with a number of endocrine agents and now is no longer
responding?
DR LOVE: Antonio, what’s your usual approach for a patient who has
been treated with a number of endocrine agents and now is no longer
responding?
![]() DR WOLFF: This is a situation in which I find capecitabine to be incredibly
useful, especially for someone who is asymptomatic. I find it an easy transition,
one pill to another pill. So that’s usually my approach.
DR WOLFF: This is a situation in which I find capecitabine to be incredibly
useful, especially for someone who is asymptomatic. I find it an easy transition,
one pill to another pill. So that’s usually my approach.
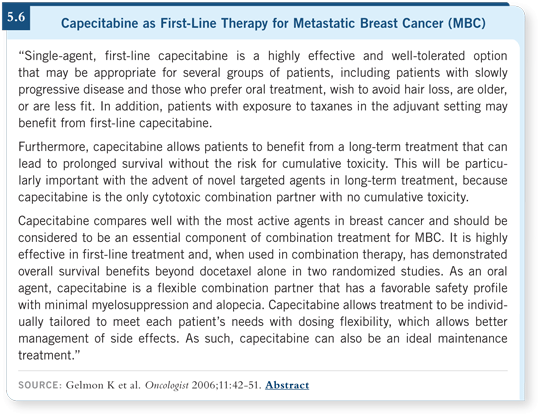
![]() DR PEREZ: I also would consider capecitabine in a situation such as this one.
Capecitabine is a well-tolerated drug (5.6). One issue, though, is which dose
and schedule to start the patient on. We never use the FDA-approved dose of
2,500 mg/m2 daily 14 days on and seven days off. We are migrating to 2,000
mg/m2 daily seven days on and seven days off or a total dose of 2,000 milligrams.
The drug is fascinating, but we still don’t know how to use it properly.
DR PEREZ: I also would consider capecitabine in a situation such as this one.
Capecitabine is a well-tolerated drug (5.6). One issue, though, is which dose
and schedule to start the patient on. We never use the FDA-approved dose of
2,500 mg/m2 daily 14 days on and seven days off. We are migrating to 2,000
mg/m2 daily seven days on and seven days off or a total dose of 2,000 milligrams.
The drug is fascinating, but we still don’t know how to use it properly.
![]() DR LOVE: Antonio, you are evaluating this in a study.
DR LOVE: Antonio, you are evaluating this in a study.
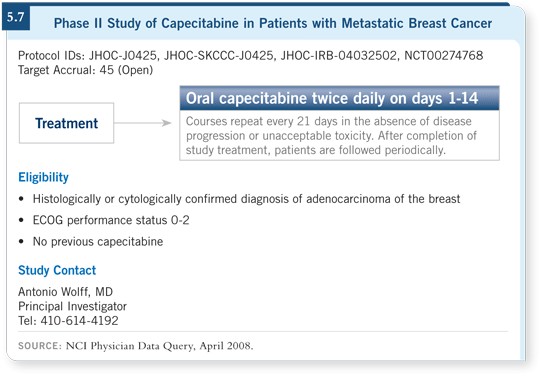
![]() DR WOLFF: Yes, we started a clinical trial about two and a half years ago.
We are using a fixed dose of capecitabine of three grams per day 14 days on,
seven days off, regardless of the patient’s size (5.7). We’re conducting intensive
pharmacokinetic and pharmacogenetic studies.
DR WOLFF: Yes, we started a clinical trial about two and a half years ago.
We are using a fixed dose of capecitabine of three grams per day 14 days on,
seven days off, regardless of the patient’s size (5.7). We’re conducting intensive
pharmacokinetic and pharmacogenetic studies.
![]() DR LOVE: What do you think about the seven-days-on, seven-days-off
schedule suggested by Larry Norton and others?
DR LOVE: What do you think about the seven-days-on, seven-days-off
schedule suggested by Larry Norton and others?
![]() DR WOLFF: This schedule was proposed shortly after we started our trial,
by the folks at Memorial. I don’t have personal experience with it at this
point, but I expect it’s a reasonable approach. We all have patients on chronic
capecitabine who ultimately start managing the pills themselves. They figure
out exactly how many days they need to take the drug before they have any
hand-foot symptoms.
DR WOLFF: This schedule was proposed shortly after we started our trial,
by the folks at Memorial. I don’t have personal experience with it at this
point, but I expect it’s a reasonable approach. We all have patients on chronic
capecitabine who ultimately start managing the pills themselves. They figure
out exactly how many days they need to take the drug before they have any
hand-foot symptoms.
![]() DR ROBERT: I agree with Antonio. At the end of the day, everybody has
his or her own capecitabine schedule. We’re involved in RIBBON 1 and
RIBBON 2, and I use capecitabine in those trials. We start off with the
original dose and then modify it. Outside a clinical trial, however, I tend to
use a flat dose of 3,000 milligrams on days one to 14.
DR ROBERT: I agree with Antonio. At the end of the day, everybody has
his or her own capecitabine schedule. We’re involved in RIBBON 1 and
RIBBON 2, and I use capecitabine in those trials. We start off with the
original dose and then modify it. Outside a clinical trial, however, I tend to
use a flat dose of 3,000 milligrams on days one to 14.
EDITOR'S NOTE
Bev, BETH, BEATRICE and the next big moment in oncology
research: NSABP-C-08
Neil Love, MD
- Select publication
INTERVIEWS
Daniel F Hayes, MD
- Select publications
John F Forbes, MD
- Select publications
Julie R Gralow, MD
- Select publications
John F R Robertson,
MB, ChB, BSc, MD
- Select publications
Roundtable Discussion
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity